Deficiency of LncRNA-CIRBIL promotes J-wave syndrome by enhancing transmural heterogeneity of Ito current: LncCIRBIL regulates J-wave syndrome via UPF1
doi: 10.1515/fzm-2025-0019
-
Abstract:
Background Transmural heterogeneity of the transient outward potassium current (Ito) is a major contributor to J-wave syndrome (JWS). However, the underlying molecular mechanisms remain elusive. The present study aimed to investigate the role of cardiac injury-related bclaf1-interacting lncRNA (lncCIRBIL) in JWS and to delineate the molecular mechanisms. Methods Whole-cell patch-clamp techniques were used to record ionic currents and action potentials (APs). Protein and mRNA expression related to Ito current were assessed. RNA immunoprecipitation, RNA Pulldown, mRNA stability, and decapping assays were performed to dissect the underlying mechanisms. Results Plasma lncCIRBIL levels were significantly reduced in JWS patients and cold-induced JWS mice. Knockout of lncCIRBIL increased the incidence of J-wave and the susceptibility to ventricular arrhythmia in mice. In lncCIRBIL-deficient mice, the transmural gradient of Kv4.2 expression and Ito current density was markedly enhanced in the right ventricle, but not the left ventricle. In contrast, cardiomyocyte-specific transgenic overexpression of lncCIRBIL produced the opposite effects. In human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), the conserved human homologous fragment of lncCIRBIL (hcf-CIRBIL) suppressed Ito, attenuated the AP notch, and prolonged APD20. Mechanistically, lncCIRBIL directly binds to up-frameshift protein1 (UPF1), promoting KCND2 mRNA decay by enhancing its decapping. Conclusions LncCIRBIL modulates the transmural heterogeneity of KCND2 expression by regulating UPF1-mediated mRNA decay. Inhibition of lncCIRBIL exacerbates JWS by enhancing right ventricular Ito heterogeneity, whereas its overexpression exerts protective effects. These findings identify lncCIRBIL as a potential therapeutic target for J-wave syndrome. -
Key words:
- long noncoding RNA /
- J-wave syndrome /
- KCND2 /
- up-frameshift protein1 /
- arrhythmia
-
Figure 1. Surface ECGs showing arrhythmias in WT and Cardiac lncCIRBIL knockout mice
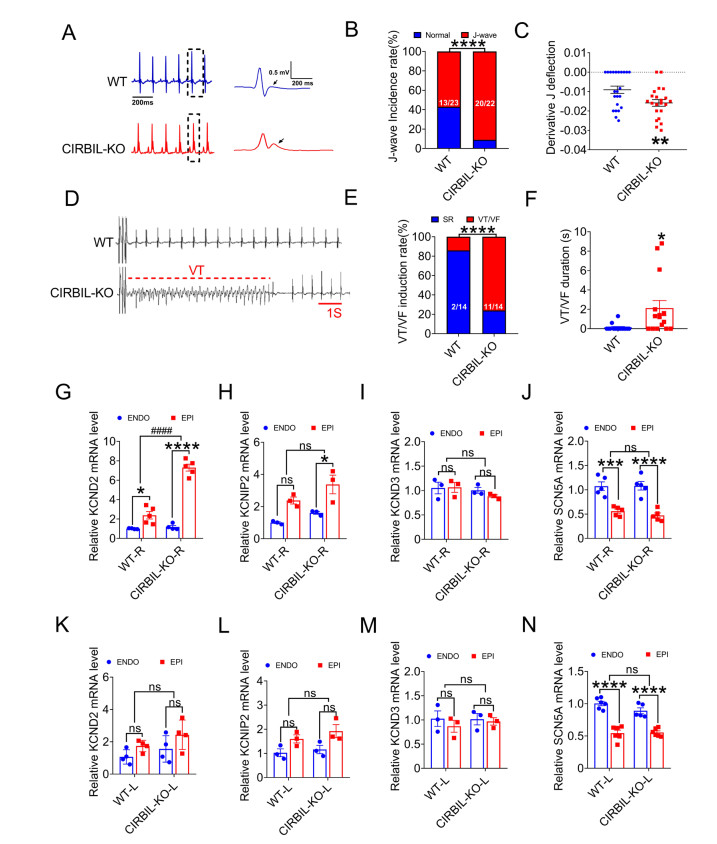
(A) Representative lead II surface ECGs from WT and CIRBIL-KO mice. Right panels show magnified boxed regions highlighting typical J-waves (arrows). (B) Incidence of J-waves in lead II of WT (N = 23) and CIRBIL-KO (N = 22) mice. ****P < 0.0001 versus WT mice. Fisher's exact test. (C) Average values of the derivative of J-wave downward deflection in lead II of WT (N = 23) and CIRBIL-KO (N = 22) mice. **P < 0.01 versus WT, unpaired t test. (D) Representative example of VT induced by programmed. stimulation. (E, F) Induction rate and duration of VT/VF following programmed stimulation in WT and CIRBIL-KO mice (N = 14 per group). *P < 0.05, ****P < 0.0001 versus WT, Fisher's exact test and unpaired t test. (G-N) mRNA expression levels of KCND2, KCNIP2, KCND3, and SCN5A in EPI and ENDO cardiomyocytes of WT-R, CIRBIL-KO-R and WT-L, CIRBIL-KO-L from WT and CIRBIL-KO mice (N = 3-6 per group). *P < 0.05, ***P < 0.001, ****P < 0.0001, ####P < 0.0001, two-way ANOVA followed by Bonferroni's multiple comparisons test. Data are expressed as mean ± SEM. Data are expressed as mean ± SEM. Electrocardiograms, ECGs; WT, Wild Type; CIRBIL-KO, CIRBIL Knockout; HR, Heart Rate; WT-R, Right Ventricle of WT; CIRBIL-KO-R, Right Ventricle of CIRBIL-KO; WT-L, Left Ventricle of WT; CIRBIL-KO-L, Left Ventricle of CIRBIL-KO; ENDO, Endocardial Cardiomyocytes; EPI, Epicardial Cardiomyocytes.
Figure 2. Effects of lncCIRBIL knockout on transmural differences of Ito and APD in the right ventricle
(A-C) Representative current traces and I-V relationships of Ito in EPI and ENDO myocytes of right ventricle from WT (N = 8) and CIRBIL-KO (N = 9) mice. *P < 0.05, **P < 0.01, ****P < 0.0001 versus ENDO; two-way ANOVA followed by Bonferroni's post hoc test. (D-F) Kv4.2 protein levels in EPI and ENDO myocytes from WT-R and CIRBIL-KO-R mice (N = 5 per group). *P < 0.05, ***P < 0.001, ****P < 0.0001 versus ENDO; two-way ANOVA followed by Bonferroni's post hoc test. (G) Representative AP recordings from EPI and ENDO myocytes of WT-R and CIRBIL-KO-R mice. (H-P) Quantitative analyses of AP parameters, including APD20, APD50, APD90, APA, RP, and OS. Quantitative data are presented as mean ± SEM. * P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 versus ENDO; two-way ANOVA with Bonferroni's post hoc test. WT, Wild Type; CIRBIL-KO, CIRBIL Knockout; WT-R, Right Ventricle of WT; CIRBIL-KO-R, Right Ventricle of CIRBIL-KO; EPI, Epicardial Cardiomyocytes; ENDO, Endocardial Cardiomyocytes; APD, Action Potential Duration; APA, Action Potential Amplitude; RP, Resting Membrane Potential; OS, Overshoot.
Figure 3. Cardiomyocyte-specific transgenic overexpression of lncCIRBIL attenuates J-wave syndrome
(A) Representative lead II surface ECGs from WT and CIRBIL-TG mice. Right panels show magnified boxed regions highlighting reduced J-wave amplitude (arrows) in CIRBIL-TG mice. (B) Incidence of J-waves in lead II of WT (N = 23) and CIRBIL-TG (N = 21) mice. ***P < 0.001 versus WT, Fisher's exact test. (C) Average values of the derivative of J-wave downward deflection in lead II of WT (N = 23) and CIRBIL-TG (N = 21) mice. **P < 0.01 versus WT mice; unpaired t test. (D) Representative Ito traces from EPI and ENDO cardiomyocytes of the right ventricle. (E, F) I-V relationships of Ito in right ventricular cardiomyocytes from WT (N = 15) and CIRBIL-TG (N = 17) mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus ENDO; two-way ANOVA followed by Bonferroni's post hoc test. (G) Kv4.2 protein expression in EPI and ENDO myocytes of WT-R and CIRBIL-TG-R mice (N = 3 per group). **P < 0.01, #P < 0.05. (H) KCND2 mRNA levels in EPI and ENDO myocytes of WT-R and CIRBIL-TG-R mice (N = 5-6 per group). ****P < 0.0001, ####P < 0.0001. (I) Typical APD traces from EPI and ENDO cardiomyocytes of WT-R and CIRBIL-TG-R mice. (J-O) Quantitative analyses of APD20, APD50, APD90, APA, RP, and OS in WT and CIRBIL-TG right ventricular cardiomyocytes (N = 17 per group). **P < 0.01, ***P < 0.001; two-way ANOVA with Bonferroni's post hoc test. Data are presented as mean ± SEM. WT, Wild Type; CIRBIL-TG, lncCIRBIL Transgenic Mice; WT-R, Right Ventricle of WT; CIRBIL-TG-R, Right Ventricle of CIRBIL-TG; ENDO, Endocardial Cardiomyocytes; EPI, Epicardial Cardiomyocytes; APD, Action Potential Duration; APA, Action Potential Amplitude; RP, Resting Potential; OS, Overshoot.
Figure 4. LncCIRBIL binds to UPF1 and regulates KCND2 mRNA stability
(A) Western blot analysis of UPF1 pulled down by lncCIRBIL. (B) RNA immunoprecipitation (RIP) showing enrichment of lncCIRBIL using UPF1 antibody in mouse heart tissue (N = 3 per group). *P < 0.05 versus IgG group, unpaired t test. (C) KCND2 mRNA decay in neonatal mouse ventricular myocytes after UPF1 knockdown. Cells were treated with 5 μg/mL actinomycin D (ActD) for the indicated times; RNA levels were quantified by RT-qPCR. N = 3 independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.001 versus NC, student's t test. (D, E) Effects of lncCIRBIL silencing or overexpression on KCND2 mRNA decay. N = 3 independent experiments. ***P < 0.001, ****P < 0.0001 versus NC, student's t test. (F, G) RIP showing enrichment of KCND2 mRNA with UPF1 antibody in cardiac tissue from WT and CIRBIL-KO mice (N = 3 per group). *P < 0.05 versus IgG group, one-way ANOVA followed by Bonferroni's post hoc test. (H) Schematic illustration of S1m-plasmid constructs with or without lncCIRBIL. (I) RNA pulldown showing interaction between lncCIRBIL and KCND2 mRNA. N = 3 independent experiments. *P < 0.05 versus S1m control, student's t test. (J) RNA pulldown assay showing lncCIRBIL-KCND2 interaction after UPF1 knockdown (N = 3 independent experiments). (K) Decapping of KCND2 mRNA in hearts of WT, CIRBIL-KO, and CIRBIL-TG mice, measured by qRT-PCR (N = 3 per group). **P < 0.01 for CIRBIL-KO versus WT; ####P < 0.0001 for CIRBIL-TG versus WT; one-way ANOVA followed by Bonferroni post hoc test. (L) UPF1 protein levels in EPI and ENDO myocytes of WT-R and CIRBIL-KO-R mice (N = 3 per group). Data are presented as mean ± SEM. WT, Wild Type; CIRBIL-KO, CIRBIL Knockout Mice; CIRBIL-TG, CIRBIL Transgenic Overexpression Mice; WT-R, Right Ventricle of Wild Type Mice; CIRBIL-TG-R, Right Ventricle of CIRBIL Transgenic Overexpression Mice; AD, ActD, Actinomycin; EPI, Epicardial Cardiomyocytes; ENDO, Endocardial Cardiomyocytes; NC, Negative Control.
Figure 5. Binding of hcf-CIRBIL to UPF1 and its influence on KCND2 in AC16 cells
Plasma levels of hcf-CIRBIL in patients with J-wave syndrome (N = 10) and healthy controls (N = 11), measured by qRT-PCR. *P < 0.05 versus Ctl, unpaired t test. (B) Pulldown assays showing binding of UPF1 by hcf-CIRBIL in mouse heart tissue and human AC16 cells. (C) Effects of hcf-CIRBIL overexpression on Kv4.2 protein and KCND2 mRNA expression in AC16 cells. qRT-PCR: N = 4; western blot: N = 3; each from 3 independent experiments. *P < 0.05, **P < 0.01 versus negative control (NC), Student's t test. (D) Effects of hcf-CIRBIL knockdown on Kv4.2 protein and KCND2 mRNA expression in AC16 cells. N = 3 independent experiments. *P < 0.05 versus NC, Student's t test. Data are expressed as mean ± SEM. hcf-CIRBIL, Human Conserved Fragment of lncCIRBIL; NC, Negative Control; Ctl, Control Subjects.
Figure 6. Effects of hcf-CIRBIL on KCND2 expression and function in hiPSC-derived cardiomyocytes (hiPSC-CMs)
(A, B) Effects of hcf-CIRBIL knockdown on Ito in hiPSC-CM (N = 10 cells per group). *P < 0.05, **P < 0.01 versus NC; two-way ANOVA comparison followed by Bonferroni's post hoc test. (C-E) Effects of hcf-CIRBIL(SI) knockdown (siRNA, SI) on notching incidence rate and AP notch depth in hiPSC-CM (N = 10 cells per group). *P < 0.05 versus NC., by student's t-test. Notching incidence was compared using Fisher's exact test. ****P < 0.0001 versus NC. (F) Effects of hcf-CIRBIL knockdown on APD20 (N = 10 cells per group). *P < 0.05 versus NC, student's t-test. (G, H) Effects of hcf-CIRBIL overexpression on Ito in hiPSC-CM. *P < 0.05, **P < 0.01, ***P < 0.001 versus NC; two-way ANOVA with Bonferroni's post hoc test. (I-L) Effects of hcf-CIRBIL overexpression on notching incidence rate and AP notch depth in hiPSC-CM (N = 10 cells per group). *P < 0.05 versus NC, student's t-test. Notching incidence compared by Fisher's exact test. ****P < 0.0001 versus NC. (M) Effects of hcf-CIRBIL overexpression on decay half-life of KCND2 mRNA in hiPSC-CM treated with 5 ug/ml ActD (N = 3 independent experiments). *P < 0.05 for hcf-CIRBIL(OE) vs NC, student's t-test. Data are expressed as mean ± SEM. hcf-CIRBIL, Human Conserved Fragment of lncCIRBIL; hiPSC-CM, Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte; NC, Negative Control; SI, Small Interfering RNA Knockdown; OE, Overexpression; APD20, Action Potential Duration at 20% Repolarization; ActD, Actinomycin D.
Figure 7. LncCIRBIL overexpression suppresses cold-induced J-point elevation and ventricular arrhythmia susceptibility
(A) Plasma lncCIRBIL levels in mice maintained at 4 ± 1 ℃ (N = 21) versus 25 ± 1 ℃ (N = 22), measured by qRT-PCR. ***P < 0.001 versus WT 25℃±1 ℃, unpaired t test. (B-D) Representative lead II ECGs from WT and CIRBIL-TG mice exposed to 25 ± 1 ℃ or 4 ± 1 ℃, and incidence of J-wave elevation. N = 23 for WT mice maintained at 4℃ ± 1 ℃, N = 23 for WT mice maintained at 25 ℃ ± 1 ℃. ****P < 0.0001 versus WT 25℃±1 ℃, ****P < 0.0001 versus WT 4 ℃ ± 1 ℃, Fisher's exact test. (E-G) Representative ECGs and incidence of VT/VF induced by programmed stimulation (N = 10 mice per group). VF/VT inducibility was compared by Fisher's exact test. ****P < 0.0001 versus WT 25 ℃ ± 1 ℃, ****P < 0.0001 versus WT at 4 ℃. (H-J) Kv4.2 protein and KCND2 mRNA expression in epicardial (EPI) and endocardial (ENDO) myocytes of right ventricles from WT mice exposed to 25 ℃ or 4 ℃. qRT-PCR: N = 6; western blot: N = 3. *P < 0.05, ***P < 0.001 versus WT-R-ENDO, #P < 0.05 versus WT-R 25℃; two-way ANOVA with Bonferroni's post hoc test. Data are expressed as mean ± SEM. WT, Wild Type; CIRBIL-TG, lncCIRBIL Transgenic Mice; WT-R, Right Ventricle of WT; CIRBIL-TG-R, Right Ventricle of CIRBIL-TG; EPI, Epicardial Cardiomyocytes; ENDO, Endocardial Cardiomyocytes; VT, Ventricular Tachycardia; VF, Ventricular Fibrillation.
Figure 8. Proposed mechanism by which lncCIRBIL regulates J-wave syndrome
LncCIRBIL binds and recruits UPF1 to promote KCND2 mRNA decay in cardiomyocytes. Reduction of lncCIRBIL impairs UPF1-mediated degradation of KCND2 mRNA, leading to increased Ito density and enhanced transmural heterogeneity in the right ventricle, which predisposes to J-wave syndrome. UPF1, up-frameshift protein1; lncCIRBIL, Injury-Related Bclaf1-Interacting LncRNA. The figure was drawn by the BioRender (Toronto, Canada).
-
[1] Macfarlane P W, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol, 2015; 66(4): 470-477. doi: 10.1016/j.jacc.2015.05.033 [2] Antzelevitch C, Yan G X. J wave syndromes. Heart Rhythm, 2010; 7(4): 549-558. doi: 10.1016/j.hrthm.2009.12.006 [3] Antzelevitch C, Yan G X. J-wave syndromes: brugada and early repolarization syndromes. Heart Rhythm, 2015; 12(8): 1852-1866. doi: 10.1016/j.hrthm.2015.04.014 [4] Yan G X, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation, 1996; 93(2): 372-379. doi: 10.1161/01.CIR.93.2.372 [5] Remme C A, Verkerk A O, Hoogaars W M, et al. The cardiac sodium channel displays differential distribution in the conduction system and transmural heterogeneity in the murine ventricular myocardium. Basic Res Cardiol, 2009; 104(5): 511-522. doi: 10.1007/s00395-009-0012-8 [6] Kim S H, Kim D Y, Kim H J, et al. Early repolarization with horizontal ST segment may be associated with aborted sudden cardiac arrest: a retrospective case control study. BMC Cardiovasc Disord, 2012; 12: 122. doi: 10.1186/1471-2261-12-122 [7] Lee W S, Nam G B, Kim S H, et al. ECG features and proarrhythmic potentials of therapeutic hypothermia. Heart, 2016; 102(19): 1558-1565. doi: 10.1136/heartjnl-2015-308821 [8] Ponnusamy M, Liu F, Zhang Y H, et al. Long noncoding RNA CPR (Cardiomyocyte Proliferation Regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation, 2019; 139(23): 2668-2684. doi: 10.1161/CIRCULATIONAHA.118.035832 [9] Gao R, Wang L, Bei Y, et al. Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation, 2021; 144(4): 303317. doi: 10.1161/CIRCULATIONAHA.120.050446 [10] Du J, Li Z, Wang X, et al. Long noncoding RNA TCONS-00106987 promotes atrial electrical remodelling during atrial fibrillation by sponging miR-26 to regulate KCNJ2. J Cell Mol Med, 2020; 24(21): 12777-12788. doi: 10.1111/jcmm.15869 [11] Zhang Y, Sun L, Xuan L, et al. Long non-coding RNA CCRR controls cardiac conduction via regulating intercellular coupling. Nat Commun, 2018; 9(1): 4176. doi: 10.1038/s41467-018-06637-9 [12] Babapoor-Farrokhran S, Gill D, Rasekhi R T. The role of long noncoding RNAs in atrial fibrillation. Heart Rhythm, 2020; 17(6): 10431049. doi: 10.1016/j.hrthm.2020.01.015 [13] Bertaso F, Sharpe C C, Hendry B M, et al. Expression of voltage-gated K + channels in human atrium. Basic Res Cardiol, 2002; 97(6): 424-433. doi: 10.1007/s00395-002-0377-4 [14] Yang B, Lin H, Xiao J, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med, 2007; 13(4): 486-491. doi: 10.1038/nm1569 [15] Yeh C F, Chang Y E, Lu C Y, et al. Expedition to the missing link: long noncoding RNAs in cardiovascular diseases. J Biomed Sci, 2020; 27(1): 48. doi: 10.1186/s12929-020-00647-w [16] Zhang Y, Zhang X, Cai B, et al. The long noncoding RNA lncCIRBIL disrupts the nuclear translocation of Bclaf1 alleviating cardiac ischemiareperfusion injury. Nat Commun, 2021; 12(1): 522. doi: 10.1038/s41467-020-20844-3 [17] Behr E R. J-wave syndromes, SCN5A, and cardiac conduction reserve: two sides of the same coin? J Am Coll Cardiol, 2021; 78(16): 1618-1620. doi: 10.1016/j.jacc.2021.09.003 [18] Antzelevitch C, Di Diego J M. J wave syndromes: what's new? Trends Cardiovasc Med, 2021; 32(6): 350-363. doi: 10.1016/j.tcm.2021.07.001 [19] He F, Jacobson A. Control of mRNA decapping by positive and negative regulatory elements in the Dcp2 C-terminal domain. RNA, 2015; 21(9): 1633-1647. doi: 10.1261/rna.052449.115 [20] Long Q Q, Wang H, Gao W, et al. Long noncoding RNA Kcna2 antisense RNA contributes to ventricular arrhythmias via silencing kcna2 in rats with congestive heart failure. J Am Heart Assoc, 2017; 6(12): E005965. doi: 10.1161/JAHA.117.005965 [21] Badri M, Patel A, Yan G X. Cellular and ionic basis of J-wave syndromes. Trends Cardiovasc Med, 2015; 25(1): 12-21. doi: 10.1016/j.tcm.2014.09.003 [22] Veerman C C, Podliesna S, Tadros R, et al. The brugada syndrome susceptibility gene HEY2 modulates cardiac transmural ion channel patterning and electrical heterogeneity. Circ Res, 2017; 121(5): 537-548. doi: 10.1161/CIRCRESAHA.117.310959 [23] Kim K H, Oh Y, Liu J, et al. Irx5 and transient outward K(+) currents contribute to transmural contractile heterogeneities in the mouse ventricle. Am J Physiol Heart Circ Physiol, 2022; 322(5): H725-H741. doi: 10.1152/ajpheart.00572.2021 [24] Gaborit N, Le Bouter S, Szuts V, et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol, 2007; 582(Pt 2): 675-693. doi: 10.1113/jphysiol.2006.126714 [25] Bridges M C, Daulagala A C, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol, 2021; 220(2): E202009045. doi: 10.1083/jcb.202009045 [26] Hwang H J, Park Y, Kim Y K. UPF1: from mRNA surveillance to protein quality control. Biomedicines, 2021; 9(8): 995. doi: 10.3390/biomedicines9080995 [27] Kim Y K, Maquat L E. UPFront and center in RNA decay: UPF1 in nonsense-mediated mRNA decay and beyond. RNA, 2019; 25(4): 407422. doi: 10.1261/rna.070136.118 [28] Gibbs M R, Chanfreau G F. UPF1 adds an m(6)A feather to its (de) cap. Cell Rep, 2022; 39(8): 110898. doi: 10.1016/j.celrep.2022.110898 [29] Yoon J H, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol, 2013; 425(19): 3723-3730. doi: 10.1016/j.jmb.2012.11.024 [30] Gong C, Maquat L E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3' UTRs via Alu elements. Nature, 2011; 470(7333): 284-288. doi: 10.1038/nature09701 [31] Guo W, Liu S, Cheng Y, et al. ICAM-1-Related noncoding RNA in cancer stem cells maintains ICAM-1 expression in hepatocellular carcinoma. Clin Cancer Res, 2016; 22(8): 2041-2050. doi: 10.1158/1078-0432.CCR-14-3106 [32] Damas N D, Marcatti M, Come C, et al. SNHG5 promotes colorectal cancer cell survival by counteracting STAU1-mediated mRNA destabilization. Nat Commun, 2016; 7: 13875. doi: 10.1038/ncomms13875 [33] Lee H C, Kang D, Han N, et al. A novel long noncoding RNA Linc-ASEN represses cellular senescence through multileveled reduction of p21 expression. Cell Death Differ, 2020; 27(6): 1844-1861. doi: 10.1038/s41418-019-0467-6 [34] Yamaki M, Sato N, Imanishi R, et al. Low room temperature can trigger ventricular fibrillation in J wave syndromes. HeartRhythm Case Rep, 2016; 2(4): 347-350. doi: 10.1016/j.hrcr.2016.04.003 -
 fzm-5-3-157_ESM.docx
fzm-5-3-157_ESM.docx

-


 投稿系统
投稿系统


 下载:
下载: