Cordycepin ameliorates brown adipose tissue whitening induced by long-term continuous light exposure via the AMPK/PGC-1α/UCP1 signaling pathway
doi: 10.1515/fzm-2025-0016
-
Abstract:
Background Long-term exposure to light has emerged as a novel risk factor for metabolic diseases. The whitening of brown adipose tissue (BAT) may play an important role in metabolic disorders caused by long-term continuous light exposure. This study aimed to investigate the morphological and functional alterations in BAT under continuous light conditions and to identify traditional Chinese medicine compounds capable of reversing these changes. Methods A metabolic disorder model was established by subjecting mice to continuous light exposure for 5 weeks. During this period, body weight, food intake, and body fat percentage were monitored. Serum levels of triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) were measured to assess lipid metabolism. Histological changes in BAT were examined using H&E staining. The expression of the thermogenic marker uncoupling protein 1 (UCP1) in BAT was determined by RT-qPCR and Western blot to evaluate thermogenic function. RNA sequencing (RNA-seq) was employed to identify differentially expressed genes (DEGs) involved in BAT whitening induced by prolonged continuous light exposure. DEGs were analyzed using the connectivity map (CMap) database to identify potential preventive and therapeutic compounds. The therapeutic efficacy of the selected compounds was subsequently evaluated using the above indicators, and key pathways were validated through western blot analysis. Results After 5 weeks of continuous light exposure, mice exhibited increased body fat percentage and serum levels of TG, impaired mitochondrial function, reduced thermogenic capacity, and whitening of BAT. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) enrichment analyses indicated that BAT whitening was primarily associated with the adenosine 5'-monophosphate-activated protein kinase (AMPK) signaling pathway, fatty acid metabolism, and circadian rhythm. Ten hub genes identified using Cytoscape were mainly related to AMPK signaling and heat shock proteins. In vivo experiments showed that cordycepin significantly attenuated the increase in body fat percentage caused by prolonged light exposure. This effect was mediated by activation of the AMPK/PGC-1α/UCP1 signaling pathway, which restored the multilocular morphology and thermogenic function of BAT. Conclusion Cordycepin mitigates continuous light-induced BAT whitening and metabolic disturbances by activating the AMPK signaling pathway. -
Key words:
- long-term continuous light /
- brown adipose tissue /
- whitening /
- cordycepin /
- AMPK
-
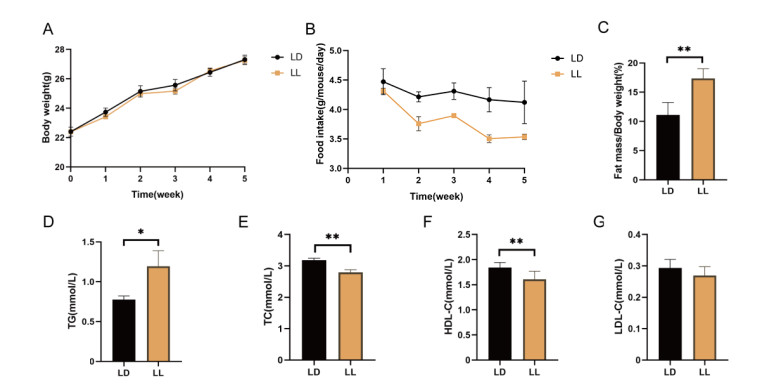
Figure 1. Continuous light exposure induces lipid metabolism disorders in mice
(A) Body weight; (B) Food intake; (C) Body fat percentage; (D) Serum levels of triglyceride (TG); (E) Total cholesterol (TC); (F) High density lipoprotein cholesterol (HDL-C); (G) Low density lipoprotein cholesterol (LDL-C). Data are presented as mean ± SEM; N = 10/group. *P < 0.05, **P < 0.01 vs. LD. LD, control group; LL, experimental group.
Figure 2. Continuous light exposure induces brown adipose tissue (BAT) whitening in mice
(A) Hematoxylin and Eosin (H&E) staining; (B) Mfn1, Mfn2, Drp1, Fis1, Pgc-1α, Ucp1 mRNA levels; (C-D) western blot for uncoupling protein 1. N = 5-6/group; *P < 0.05, **P < 0.01 vs. LD. BAT, brown adipose tissue; UCP1, uncoupling protein 1.
Figure 4. Protein-protein interaction (PPI) network construction and hub genes screening
(A) PPI network of differentially expressed genes (DEGs); (B) Hub genes of DEGs; (C) Kyoto encyclopedia of genes and genomes (KEGG) analysis of hub genes; (D) Heat maps of hub genes; (E) mRNA levels of hub genes. N = 5-6/group; *P < 0.05, **P < 0.01 vs. LD. LD, control group.
Figure 5. Cordycepin activates brown adipose tissue through the adenosine 5'-monophosphate-activated protein kinase (AMPK)/PGC-1α/uncoupling protein 1 (UCP1) pathway
(A) Body weight; (B) Food intake; (C) Fat mass; (D) Serum levels of triglyceride (TG); (E) Total cholesterol (TC); (F) High density lipoprotein (HDL-C); (G) Low density lipoprotein (LDL-C); (H) H&E staining; (I) Ucp1 mRNA levels; (J) Pgc-1α mRNA levels; (K-N) Western blot for AMPK, p-AMPK, UCP1 and PGC-1α. N = 5-6/group; *P < 0.05, **P < 0.01 vs. LD; #P < 0.05, ##P < 0.01 vs. LL. LD, control group; LL, experimental group.
Table 1. List of primers used for real-time quantitative PCR
Gene Forward Primer (5′-3′) Reverse Primer (5′-3′) Mfn1 GATAAAGTCCTCCCCAGCGG GCATGGGCCAGCTGATTAAC Mfn2 TCCTCTCCCTCTGACACCTG AAGGAGAGGGCGATGAGTCT Drp1 GCCTCAGATCGTCGTAGTGG TCAACTCCATTTTCTTCTCCTGT Fis1 AGAGACGAAGCTGCAAGGAAT CCGCTGTTCCTCTTTGCTCC Ucp1 TCAGGATTGGCCTCTACGAC CTGTAGGCTGCCCAATGAAC Pgc1-α AGTGGTGTAGCGACCAATCG GGGCAATCCGTCTTCATCCA Stip1 ACCTGGGCACGAAACTACAG GGCATCATTTCCCAGCTCCT Hsph1 ATCTTCACCATCTCCACGGC TTCTTGGCTTCTGGAGGCTG Mtor GGCCCAGGCAGAAAACTTAC GCGCTCTGCTCCTTGATTCT Prkag2 GACCCTATCAGTGGGAACGC CCTGACTCATCCACCACAGG Prkab1 TTCGTGGATGGACAGTGGAC CGGGGCTTTGAACCTCTCTT Foxo1 GATAAGGGCGACAGCAACAG CTCTTGCCTCCCTCTGGATTG Bag3 CCCAACTGCTCATGGACCTG GAGCTGGGTAGTGGGTCTTC Dnajb1 ACGACGAGATCAAGCGAGC GGGGTCTCCGTGGAATGTG Hspa1b CATGGTGCTGACGAAGATGA GAGAGTCGTTGAAGTAGGCG 36B4 CGACCTGGAAGTCCAACTAC ATCTGCTGCATCTGCTTG Table 2. Traditional Chinese Medicine Compounds
Name Score resveratrol 0.5505 rotenone 0.5361 colchicine 0.5276 ellipticine 0.5112 cordycepin 0.4984 enoxolone 0.4771 pterostilbene 0.4736 scoulerine 0.4622 paclitaxel 0.4507 evodiamine 0.4507 -
[1] Kolomeichuk SN, Korostovtseva LS, Morozov A V, et al. Comparative analysis of sleep hygiene and patterns among adolescents in two Russian Arctic regions: a pilot study. Children (Basel), 2024; 11(3): 279. [2] Thuany M, Viljoen C, Gomes T N, et al. Antarctic expeditions: a systematic review of the physiological, nutritional, body composition and psychological responses to treks across the continental ice. Sports Med, 2024; 55(5): 1145-1163. [3] Maciejczyk M, Arazny A, Opyrchal M. Changes in aerobic performance, body composition, and physical activity in polar explorers during a year-long stay at the polar station in the Arctic. Int J Biometeorol, 2017; 61(4): 669-675. doi: 10.1007/s00484-016-1244-6 [4] Xu Y J, Xie Z Y, Gong Y C, et al. The association between outdoor light at night exposure and adult obesity in Northeastern China. Int J Environ Health Res, 2024; 34(2): 708-718. doi: 10.1080/09603123.2023.2165046 [5] Sakers A, De Siqueira M K, Seale P, et al. Adipose-tissue plasticity in health and disease. Cell, 2022; 185(3): 419-446. doi: 10.1016/j.cell.2021.12.016 [6] Cedikova M, Kripnerová M, Dvorakova J, et al. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int, 2016; 2016: 6067349. doi: 10.1155/2016/6067349 [7] Lee P, Bova R, Schofield L, et al. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metabolism, 2016; 23(4): 602-609. doi: 10.1016/j.cmet.2016.02.007 [8] Kuipers E N, Held N M, In Het Panhuis W, et al. A single day of high-fat diet feeding induces lipid accumulation and insulin resistance in brown adipose tissue in mice. Am J Physiol Endocrinol Metab, 2019; 317(5): E820-E830. doi: 10.1152/ajpendo.00123.2019 [9] Pan X X, Yao K L, Yang Y F, et al. Senescent T cell induces brown adipose tissue "whitening" via secreting IFN-gamma. Front Cell Dev Biol, 2021; 9: 637424. doi: 10.3389/fcell.2021.637424 [10] Hao L, Khan M S H, Zu Y, et al. Thermoneutrality inhibits thermogenic markers and exacerbates nonalcoholic fatty liver disease in mice. Int J Mol Sci, 2024; 25(15): 8482. doi: 10.3390/ijms25158482 [11] Bolin A P, De Fatima Silva F, Salgueiro R B, et al. Glucocorticoid modulates oxidative and thermogenic function of rat brown adipose tissue and human brown adipocytes. J Cell Physiol, 2024; 239(9): 1-12. [12] Kooijman S, Van Den Berg R, Ramkisoensing A, et al. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci U S A, 2015; 112(21): 6748-6753. doi: 10.1073/pnas.1504239112 [13] Li X, Xiong X, Yi C. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat Methods, 2016; 14(1): 23-31. [14] Du K, Chen G H, Bai X, et al. Dynamics of transcriptome and chromatin accessibility revealed sequential regulation of potential transcription factors during the brown adipose tissue whitening in rabbits. Front Cell Dev Biol, 2022; 10: 981661. doi: 10.3389/fcell.2022.981661 [15] Lyu J, Liu Y, Liu F, et al. Therapeutic effect and mechanisms of traditional Chinese medicine compound (Qilong capsule) in the treatment of ischemic stroke. Phytomedicine, 2024; 132: 155781. doi: 10.1016/j.phymed.2024.155781 [16] Huang W, Yu P, Zhao X, et al. CMAP prediction and experimental validation of Forskolin as a podocyte protective and anti-proteinuric drug for nephrotoxic serum-treated mice. Biochem Pharmacol, 2025; 232: 116727. doi: 10.1016/j.bcp.2024.116727 [17] Markina N O, Matveev G A, Zasypkin G G, et al. Role of brown adipose tissue in metabolic health and efficacy of drug treatment for obesity. J Clin Med, 2024; 13(14): 4151. doi: 10.3390/jcm13144151 [18] Carpentier A C, Blondin D P, Virtanen K A, et al. Brown Adipose Tissue Energy Metabolism in Humans. Front Endocrinol (Lausanne), 2018; 9: 447. doi: 10.3389/fendo.2018.00447 [19] Li Y, Cao S, Li Y. Mechanistic study of heat shock protein 60-mediated apoptosis in DF-1 cells. Poult Sci, 2024; 103(6): 103619. doi: 10.1016/j.psj.2024.103619 [20] Zininga T, Ramatsui L, Shonhai A. Heat shock proteins as immunomodulants. Molecules, 2018; 23(11): 2846. doi: 10.3390/molecules23112846 [21] Wong S H D, Yin B, Li Z, et al. Mechanical manipulation of cancer cell tumorigenicity via heat shock protein signaling. Sci Adv, 2023; 9(27): 9593. doi: 10.1126/sciadv.adg9593 [22] Day E A, Townsend L K, Rehal S, et al. Macrophage AMPK β1 activation by PF-06409577 reduces the inflammatory response, cholesterol synthesis, and atherosclerosis in mice. iScience, 2023; 26(11): 108269. doi: 10.1016/j.isci.2023.108269 [23] Cheng Y, Mei X, Shao W, et al. Nobiletin alleviates macrophage M2 polarization by activating AMPK-mTOR-mediated autophagy in pulmonary fibrosis mice. Int Immunopharmacol, 2024; 139: 112792. doi: 10.1016/j.intimp.2024.112792 [24] Shen B, Wen Y, Li S, et al. Paeonol ameliorates hyperlipidemia and autophagy in mice by regulating Nrf2 and AMPK/mTOR pathways. Phytomedicine, 2024; 132: 155839. doi: 10.1016/j.phymed.2024.155839 [25] Wu F, Lu F, Dong H, et al. Oxyberberine inhibits hepatic gluconeogenesis via AMPK-mediated suppression of FoxO1 and CRTC2 signaling axes. Phytother Res, 2024, Online ahead of print. [26] Sagliocchi S, Schiano E, Acampora L, et al. AbaComplex enhances mitochondrial biogenesis and adipose tissue browning: implications for obesity and glucose regulation. Foods, 2024; 14(1): 48. doi: 10.3390/foods14010048 [27] Tang X, Shi Y, Chen Y, et al. Tetrahydroberberrubine exhibits preventive effect on obesity by activating PGC1α-mediated thermogenesis in white and brown adipose tissue. Biochemical pharmacology, 2024; 226: 116381. doi: 10.1016/j.bcp.2024.116381 [28] Zhang Y Q, Zhang Z Z, Zhang Y W, et al. Baicalin promotes the activation of brown and white adipose tissue through AMPK/PGC1α pathway. Eur J Pharmacol, 2022; 922: 174913. doi: 10.1016/j.ejphar.2022.174913 [29] Wang H, An Y, Rajput S A, et al. Resveratrol and (-)-epigallocatechin-3-gallate regulate lipid metabolism by activating the AMPK pathway in hepatocytes. Biology, 2024; 13(6): 368. doi: 10.3390/biology13060368 [30] Lan T, Yu Y, Zhang J, et al. Cordycepin ameliorates nonalcoholic steatohepatitis by activation of the AMP-activated protein kinase signaling pathway. Hepatology, 2021; 74(2): 686-703. doi: 10.1002/hep.31749 [31] Shen B, Wang Y, Cheng J, et al. Pterostilbene alleviated NAFLD via AMPK/mTOR signaling pathways and autophagy by promoting Nrf2. Phytomedicine: international journal of phytotherapy and phytopharmacology, 2023; 109: 154561. doi: 10.1016/j.phymed.2022.154561 [32] Gómez-garcía I, Fernández-quintela A, Portillo M P, et al. Changes in brown adipose tissue induced by resveratrol and its analogue pterostilbene in rats fed with a high-fat high-fructose diet. J Physiol Biochem, 2024; 80(3): 627-637. doi: 10.1007/s13105-023-00985-x [33] Yan H, Shao M, Lin X, et al. Resveratrol stimulates brown of white adipose via regulating ERK/DRP1-mediated mitochondrial fission and improves systemic glucose homeostasis. Endocrine, 2025; 87(1): 144-158. [34] Nagao K, Jinnouchi T, Kai S, et al. Pterostilbene, a dimethylated analog of resveratrol, promotes energy metabolism in obese rats. J Nutr Biochem, 2017; 43: 151-155. doi: 10.1016/j.jnutbio.2017.02.009 [35] Xu H, Wu B, Wang X, et al. Cordycepin regulates body weight by inhibiting lipid droplet formation, promoting lipolysis and recruiting beige adipocytes. J Pharm Pharmacol, 2019; 71(9): 1429-1439. doi: 10.1111/jphp.13127 [36] Qi G, Zhou Y, Zhang X, et al. Cordycepin promotes browning of white adipose tissue through an AMP-activated protein kinase (AMPK)-dependent pathway. Acta Pharm Sin B, 2019; 9(1): 135-143. doi: 10.1016/j.apsb.2018.10.004 [37] Meng J J, Shen J W, Li G, et al. Light modulates glucose metabolism by a retina-hypothalamus-brown adipose tissue axis. Cell, 2023; 186(2): 398-412. e17. doi: 10.1016/j.cell.2022.12.024 [38] Rao F, Xue T. Circadian-independent light regulation of mammalian metabolism. Nat Metab, 2024; 6(6): 1000-1007. doi: 10.1038/s42255-024-01051-6 -
 fzm-5-3-129_ESM.docx
fzm-5-3-129_ESM.docx

-


 投稿系统
投稿系统


 下载:
下载: