Effect of aging on cardiovascular responses to cold stress in humans
doi: 10.2478/fzm-2022-0022
-
Abstract: Cold exposure increases the risk of adverse events related to cardiovascular causes, especially in the elderly. In this review, we focus on recent findings concerning the impact of aging on the regulatory mechanisms of cold-induced cardiovascular responses. In response to cold exposure, the initial physiological thermoregulation in healthy young persons, such as cutaneous vasoconstriction to reduce heat loss, is attenuated in older individuals, resulting in a reduced ability of the older persons to maintain body temperature in cold environment. Impaired sympathetic skin response, reduced noradrenergic neurotransmitter synthesis, insufficient noradrenergic transmitters, and altered downstream signaling pathways inside the vascular smooth muscle may be among the underlying mechanisms for the maladaptive vasoconstrictive response to cold stress in the elderly. The increase in blood pressure during cold exposure in young persons may be further augmented in aging adults, due to greater central arterial stiffness or diminished baroreflex sensitivity with aging. Cold stress raises myocardial oxygen demand caused by increased afterload in both young and old adults. The elderly cannot adjust to meet the increased oxygen demand due to reduced left ventricular compliance and coronary blood flow with advancing age, rendering the elderly more susceptible to hypothermiainduced cardiovascular complications from cold-related diseases. These age-associated thermoregulatory impairments may further worsen patients' health risk with existing cardiovascular diseases such as hypertension, coronary artery disease, and heart failure. We searched PubMed for papers related to cold stress and its relationship with aging, and selected the most relevant publications for discussion.
-
Key words:
- aging /
- cardiovascular /
- cold stress /
- hypertension /
- coronary artery disease /
- heart failure
-
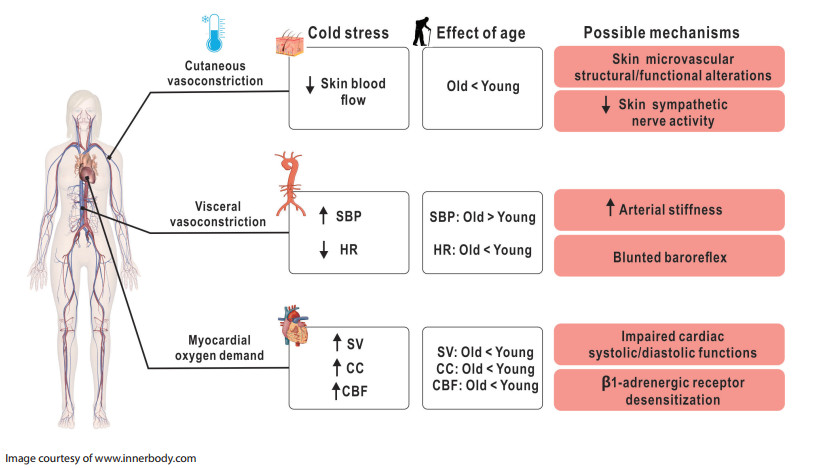
Figure 1. Summary of recent research advances in the cardiovascular response to cold stress of older adults
This figure schematically depicts the following points: (1) the cardiovascular response to cold stress in healthy young adults; (2) how these responses differ in healthy older adults; and (3) possible mechanisms mediating age-related differences. SBP, systolic blood pressure; HR, heart rate; SV, stroke volume; CC, cardiac contractility; CBF, coronary blood flow.
-
[1] The Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet, 1997; 349(9062): 1341-1346. doi: 10.1016/S0140-6736(96)12338-2 [2] Fares A. Winter cardiovascular diseases phenomenon. N Am J Med Sci, 2013; 5(4): 266-279. doi: 10.4103/1947-2714.110430 [3] Manfredini R, Manfredini F, Boari B, et al. Seasonal and weekly patterns of hospital admissions for nonfatal and fatal myocardial infarction. Am J Emerg Med, 2009; 27(9): 1097-1103. doi: 10.1016/j.ajem.2008.08.009 [4] Mehta R H, Manfredini R, Hassan F, et al. Chronobiological patterns of acute aortic dissection. Circulation, 2002; 106(9): 1110-1115. doi: 10.1161/01.CIR.0000027568.39540.4B [5] Gallerani M, Boari B, Manfredini F, et al. Seasonal variation in heart failure hospitalization. Clin Cardiol, 2011; 34(6): 389-394. doi: 10.1002/clc.20895 [6] Wang L, Liu T, Hu M, et al. The impact of cold spells on mortality and effect modification by cold spell characteristics. Sci Rep, 2016; 6(1): 38380. doi: 10.1038/srep38380 [7] Fang E F, Xie C, Schenkel J A, et al. A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res Rev, 2020; 64: 101174. doi: 10.1016/j.arr.2020.101174 [8] Stocks J M, Taylor N A, Tipton M J, et al. Human physiological responses to cold exposure. Aviat, Space, and Environ Med, 2004; 75(5): 444-457. [9] Johnson J M, Minson C T, Kellogg D L Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol, 2014; 4(1): 33-89. [10] Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol, 2010; 109(4): 1221-1228. doi: 10.1152/japplphysiol.00298.2010 [11] Castellani J W, Young A J. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton Neurosci, 2016; 196: 63-74. doi: 10.1016/j.autneu.2016.02.009 [12] Greaney J L, Kenney W L, Alexander L M. Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci, 2016; 196: 81-90. doi: 10.1016/j.autneu.2015.11.002 [13] Wilson T E, Sauder C L, Kearney M L, et al. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol, 2007; 103(4): 1257-1262. doi: 10.1152/japplphysiol.00401.2007 [14] Kingma B R, Frijns A J, Saris W H, et al. Increased systolic blood pressure after mild cold and rewarming: relation to cold-induced thermogenesis and age. Acta physiol, 2011; 203(4): 419-427. doi: 10.1111/j.1748-1716.2011.02336.x [15] Mäkinen T M, Mäntysaari M, Pääkkönen T, et al. Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Avia Space Environ Med, 2008; 79(9): 875-882. doi: 10.3357/ASEM.2235.2008 [16] Korhonen I. Blood pressure and heart rate responses in men exposed to arm and leg cold pressor tests and whole-body cold exposure. Int J Circumpolar Health, 2006; 65(2): 178-184. doi: 10.3402/ijch.v65i2.18090 [17] Keatinge W R, Coleshaw S R, Cotter F, et al. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clin Res Ed), 1984; 289(6456): 1405-1408. doi: 10.1136/bmj.289.6456.1405 [18] Khurana R K, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res, 2006; 16(3): 202-207. doi: 10.1007/s10286-006-0332-9 [19] Reyners A K, Tio R A, Vlutters F G, et al. Re-evaluation of the cold face test in humans. Eur J Appl Physiol, 2000; 82(5-6): 487-492. doi: 10.1007/s004210000217 [20] Stemper B, Hilz M J, Rauhut U, et al. Evaluation of cold face test bradycardia by means of spectral analysis. Clin Auton Res, 2002; 12(2): 78-83. doi: 10.1007/s102860200024 [21] Hess K L, Wilson T E, Sauder C L, et al. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol, 2009; 107(4): 1076-1082. doi: 10.1152/japplphysiol.00605.2009 [22] Greaney J L, Stanhewicz A E, Kenney W L, et al. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol, 2014; 117(6): 648-657. doi: 10.1152/japplphysiol.00516.2014 [23] Muller M D, Gao Z, Mast J L, et al. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol, 2012; 302(8): H1737-1746. doi: 10.1152/ajpheart.01195.2011 [24] Muller M D, Gao Z, Drew R C, et al. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol, 2011; 111(6): 1694-1702. doi: 10.1152/japplphysiol.00909.2011 [25] Jarvis S S, Okada Y, Levine B D, et al. Central integration and neural control of blood pressure during the cold pressor test: a comparison between hydrochlorothiazide and aliskiren. Physiol Rep, 2015; 3(9): e12502. doi: 10.14814/phy2.12502 [26] Siegrist P T, Gaemperli O, Koepfli P, et al. Repeatability of cold pressor test-induced flow increase assessed with H(2)(15)O and PET. J Nucl Med, 2006; 47(9): 1420-1426. [27] Hilz M J, Stemper B, Sauer P, et al. Cold face test demonstrates parasympathetic cardiac dysfunction in familial dysautonomia. Am J Physiol, 1999; 276(6): R1833-R1839. [28] Heindl S, Struck J, Wellhöner P, et al. Effect of facial cooling and cold air inhalation on sympathetic nerve activity in men. Respir Physiol Neurobiol, 2004; 142(1): 69-80. doi: 10.1016/j.resp.2004.05.004 [29] Hintsala H, Kandelberg A, Herzig K H, et al. Central aortic blood pressure of hypertensive men during short-term cold exposure. Am J Hypertens, 2014; 27(5): 656-664. doi: 10.1093/ajh/hpt136 [30] Hintsala H, Kenttä T V, Tulppo M, et al. Cardiac repolarization and autonomic regulation during short-term cold exposure in hypertensive men: an experimental study. PloS Pne, 2014; 9(7): e99973. [31] Hintsala H E, Kiviniemi A M, Tulppo M P, et al. Hypertension does not alter the increase in cardiac baroreflex sensitivity caused by moderate cold exposure. Front physiol, 2016; 7: 204. [32] Sanchez-Gonzalez M A, Figueroa A. Cold exposure attenuates post exercise cardiovagal reactivation and sympathetic withdrawal. Auton Neurosci, 2013; 176(1-2): 95-97. doi: 10.1016/j.autneu.2013.02.002 [33] Wilson T E, Crandall C G. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev, 2011; 39(1): 12-17. doi: 10.1097/JES.0b013e318201eed6 [34] Manou-Stathopoulou V, Goodwin C D, Patterson T, et al. The effects of cold and exercise on the cardiovascular system. Heart, 2015; 101(10): 808-820. doi: 10.1136/heartjnl-2014-306276 [35] Mudge G H Jr., Grossman W, Mills R M Jr, et al. Reflex increase in coronary vascular resistance in patients with ischemic heart disease. N Engl J Med, 1976; 295(24): 1333-1337. doi: 10.1056/NEJM197612092952401 [36] Muller M D, Gao Z, McQuillan P M, et al. Coronary responses to cold air inhalation following afferent and efferent blockade. Am J Physiol Heart Circ Physiol, 2014; 307(2): H228-H235. doi: 10.1152/ajpheart.00174.2014 [37] Stephens D P, Aoki K, Kosiba W A, et al. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol, 2001; 280(4): H1496-1504. doi: 10.1152/ajpheart.2001.280.4.H1496 [38] Stephens D P, Bennett L A, Aoki K, et al. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol, 2002; 282(1): H264-272. doi: 10.1152/ajpheart.2002.282.1.H264 [39] Degroot D W, Kenney W L. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Heart Circ Physiol, 2007; 292(1): R103-R108. [40] Falk B, Bar-Or O, Smolander J, et al. Response to rest and exercise in the cold: effects of age and aerobic fitness. J Appl Physiol, 1994; 76(1): 72-78. doi: 10.1152/jappl.1994.76.1.72 [41] Kenney W L, Armstrong C G. Reflex peripheral vasoconstriction is diminished in older men. J Appl Physiol, 1996; 80(2): 512-515. doi: 10.1152/jappl.1996.80.2.512 [42] Frank S M, Raja S N, Bulcao C, et al. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol Regul Integr Comp Physiol, 2000; 279(1): R349-354. doi: 10.1152/ajpregu.2000.279.1.R349 [43] Holowatz L A, Kenney W L. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol, 2010; 109(5): 1538-1544. doi: 10.1152/japplphysiol.00338.2010 [44] Grassi G, Seravalle G, Turri C, et al. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation, 2003; 108(6): 729-735. doi: 10.1161/01.CIR.0000081769.02847.A1 [45] Greaney J L, Alexander L M, Kenney W L. Sympathetic control of reflex cutaneous vasoconstriction in human aging. J Appl Physiol, 2015; 119(7): 771-782. doi: 10.1152/japplphysiol.00527.2015 [46] Kaufman S. Establishment of tetrahydrobiopterin as the hydroxylase cofactor and a review of some recent studies in man. Psychopharmacol Bull, 1978; 14(4): 38-40. [47] Moens A L, Kass D A. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol, 2007; 50(3): 238-246. doi: 10.1097/FJC.0b013e318123f854 [48] Rossman M J, Santos-Parker J R, Steward C A C, et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension, 2018; 71(6): 1056-1063. doi: 10.1161/HYPERTENSIONAHA.117.10787 [49] Seals D R, Jablonski K L, Donato A J. Aging and vascular endothelial function in humans. Clin Sci (Lond), 2011; 120(9): 357-375. doi: 10.1042/CS20100476 [50] Lang J A, Holowatz L A, Kenney W L. Local tetrahydrobiopterin administration augments cutaneous vasoconstriction in aged humans. J Physiol, 2009; 587(Pt 15): 3967-3974. [51] Lang J A, Holowatz L A, Kenney W L. Localized tyrosine or tetrahydrobiopterin supplementation corrects the age-related decline in cutaneous vasoconstriction. J Physiol, 2010; 588(Pt 8): 1361-1368. [52] Stanhewicz A E, Alexander L M, Kenney W L. Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol, 2013; 115(7): 1025-1031. doi: 10.1152/japplphysiol.00626.2013 [53] Lang J A, Smaller K A. Oral l-tyrosine supplementation augments the vasoconstriction response to whole-body cooling in older adults. Exp Physiol, 2017; 102(7): 835-844. doi: 10.1113/EP086329 [54] Lang J A, Krajek A C, Schwartz K S, et al. Oral L-tyrosine supplementation improves core temperature maintenance in older adults. Med Sci Sports Exerc, 2020; 52(4): 928-934. doi: 10.1249/MSS.0000000000002188 [55] Thompson C S, Kenney W L. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol, 2004; 558(Pt 2): 697-704. [56] Thompson C S, Holowatz L A, Kenney W L. Attenuated noradrenergic sensitivity during local cooling in aged human skin. J Physiol, 2005; 564(Pt 1): 313-319. [57] Wilson T E, Monahan K D, Short D S, et al. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol, 2004; 287(5): R1230-R1234. doi: 10.1152/ajpregu.00467.2004 [58] Lang J A, Jennings J D, Holowatz L A, et al. Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase-dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol, 2009; 297(5): H1792-H1797. doi: 10.1152/ajpheart.00509.2009 [59] Lang J A, Kolb K E. Angiotensin Ⅱ type I receptor blockade attenuates reflex cutaneous vasoconstriction in aged but not young skin. Am J Physiol Heart Circ Physiol, 2015; 308(10): H1215-H1220. doi: 10.1152/ajpheart.00017.2015 [60] Thompson-Torgerson C S, Holowatz L A, Flavahan N A, et al. Rho kinase-mediated local cold-induced cutaneous vasoconstriction is augmented in aged human skin. Am J Physiol Heart Circ Physiol, 2007; 293(1): H30-H36. doi: 10.1152/ajpheart.00152.2007 [61] Loirand G, Guérin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res, 2006; 98(3): 322-334. doi: 10.1161/01.RES.0000201960.04223.3c [62] Thapa D, Valente J S, Barrett B, et al. Dysfunctional TRPM8 signalling in the vascular response to environmental cold in ageing. ELife, 2021; 10: e70153. doi: 10.7554/eLife.70153 [63] Woodhouse P R, Khaw K T, Plummer M. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J Hypertension, 1993; 11(11): 1267-1274. [64] Wagner J A, Horvath S M. Cardiovascular reactions to cold exposures differ with age and gender. J Appl Physiol, 1985; 58(1): 187-192. doi: 10.1152/jappl.1985.58.1.187 [65] Collins K J, Easton J C, Belfield-Smith H, et al. Effects of age on body temperature and blood pressure in cold environments. Clin Sci, 1985; 69(4): 465-470. doi: 10.1042/cs0690465 [66] Collins K J, Abdel-Rahman T A, Goodwin J, et al. Circadian body temperatures and the effects of a cold stress in elderly and young subjects. Age and ageing, 1995; 24(6): 485-489. doi: 10.1093/ageing/24.6.485 [67] Inoue Y, Nakao M, Araki T, et al. Thermoregulatory responses of young and older men to cold exposure. Eur J Appl Physiol Occup Physiol, 1992; 65(6): 492-498. doi: 10.1007/BF00602354 [68] Budd G M, Brotherhood J R, Hendrie A L, et al. Effects of fitness, fatness, and age on men's responses to whole body cooling in air. J Appl Physiol, 1991; 71(6): 2387-2393. doi: 10.1152/jappl.1991.71.6.2387 [69] Edwards D G, Gauthier A L, Hayman M A, et al. Acute effects of cold exposure on central aortic wave reflection. J Appl Physiol, 2006; 100(4): 1210-1214. doi: 10.1152/japplphysiol.01154.2005 [70] Laitinen T, Hartikainen J, Vanninen E, et al. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol, 1998; 84(2): 576-583. doi: 10.1152/jappl.1998.84.2.576 [71] O'Mahony D, Bennett C, Green A, et al. Reduced baroreflex sensitivity in elderly humans is not due to efferent autonomic dysfunction. Clin Sci, 2000; 98(1): 103-110. doi: 10.1042/cs0980103 [72] Monahan K D. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol, 2007; 293(1): R3-R12. doi: 10.1152/ajpregu.00031.2007 [73] Okada Y, Galbreath M M, Shibata S, et al. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension, 2012; 59(1): 98-104. doi: 10.1161/HYPERTENSIONAHA.111.176560 [74] Edwards D G, Roy M S, Prasad R Y. Wave reflection augments central systolic and pulse pressures during facial cooling. Am J Physiol Heart Circ Physiol, 2008; 294(6): H2535-H2539. doi: 10.1152/ajpheart.01369.2007 [75] Franklin S S, Gustin W T, Wong N D, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation, 1997; 96(1): 308-315. doi: 10.1161/01.CIR.96.1.308 [76] Lakatta E G, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation, 2003; 107(1): 139-146. doi: 10.1161/01.CIR.0000048892.83521.58 [77] Tochihara Y, Yamashita K, Fujii K, et al. Thermoregulatory and cardiovascular responses in the elderly towards a broad range of gradual air temperature changes. J Therm Biol, 2021; 99: 103007. doi: 10.1016/j.jtherbio.2021.103007 [78] Juneau M, Johnstone M, Dempsey E, et al. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation, 1989; 79(5): 1015-1020. doi: 10.1161/01.CIR.79.5.1015 [79] Wilson T E, Cui J, Zhang R, et al. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. Journal of applied physiology, 2002; 93(1): 85-91. doi: 10.1152/japplphysiol.01043.2001 [80] Wilson T E, Tollund C, Yoshiga C C, et al. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol, 2007; 585(Pt 1): 279-285. [81] Cui J, Durand S, Crandall C G. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J Appl Physiol, 2007; 103(4): 1284-1289. doi: 10.1152/japplphysiol.00115.2007 [82] Yamazaki F, Sone R. Modulation of arterial baroreflex control of heart rate by skin cooling and heating in humans. J Appl Physiol, 2000; 88(2): 393-400. doi: 10.1152/jappl.2000.88.2.393 [83] Thompson-Torgerson C S, Holowatz L A, Kenney W L. Altered mechanisms of thermoregulatory vasoconstriction in aged human skin. Exerc Sport Sci Rev, 2008; 36(3): 122-127. doi: 10.1097/JES.0b013e31817bfd47 [84] Wilson T E, Gao Z, Hess K L, et al. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol, 2010; 298(6): R1627-R1633. doi: 10.1152/ajpregu.00099.2010 [85] Tighe D A, Vinch C S, Hill J C, et al. Influence of age on assessment of diastolic function by Doppler tissue imaging. Am J Cardiol, 2003; 91(2): 254-257. doi: 10.1016/S0002-9149(02)03122-3 [86] Prasad A, Popovic Z B, Arbab-Zadeh A, et al. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol, 2007; 99(12): 1629-1636. doi: 10.1016/j.amjcard.2007.01.050 [87] Innelli P, Sanchez R, Marra F, et al. The impact of aging on left ventricular longitudinal function in healthy subjects: a pulsed tissue Doppler study. Eur J Echocardiogr, 2008; 9(2): 241-249. [88] Arbab-Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation, 2004; 110(13): 1799-1805. doi: 10.1161/01.CIR.0000142863.71285.74 [89] Zeiher A M, Drexler H, Wollschlaeger H, et al. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol, 1989; 14(5): 1181-1190. doi: 10.1016/0735-1097(89)90414-2 [90] Rowe G, Tracy E, Beare J E, et al. Cell therapy rescues aginginduced beta-1 adrenergic receptor and GRK2 dysfunction in the coronary microcirculation. GeroScience, 2022; 44(1): 329-348. doi: 10.1007/s11357-021-00455-6 [91] Rowe G, Kelm N Q, Beare J E, et al. Enhanced beta-1 adrenergic receptor responsiveness in coronary arterioles following intravenous stromal vascular fraction therapy in aged rats. Aging, 2019; 11(13): 4561-4578. doi: 10.18632/aging.102069 [92] Sun Z. Cardiovascular responses to cold exposure. Front Biosci (Elite edition), 2010; 2(2): 495-503. [93] Safar M E. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol, 2018; 15(2): 97-105. doi: 10.1038/nrcardio.2017.155 [94] Greaney J L, Kenney W L, Alexander L M. Sympathetic function during whole body cooling is altered in hypertensive adults. J Appl Physiol, 2017; 123(6): 1617-1624. doi: 10.1152/japplphysiol.00613.2017 [95] Greaney J L, Kenney W L, Alexander L M. Neurovascular mechanisms underlying augmented cold-induced reflex cutaneous vasoconstriction in human hypertension. J Physiol, 2017; 595(5): 1687-1698. doi: 10.1113/JP273487 [96] Zalewski P, Buszko K, Zawadka-Kunikowska M, et al. Cardiovascular and autonomic responses to whole-body cryostimulation in essential hypertension. Cryobiol, 2014; 69(2): 249-255. doi: 10.1016/j.cryobiol.2014.07.014 [97] Gao Z, Wilson T E, Drew R C, et al. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol, 2012; 302(1): H312-H318. doi: 10.1152/ajpheart.00297.2011 [98] Meyer P, Guiraud T, Curnier D, et al. Exposure to extreme cold lowers the ischemic threshold in coronary artery disease patients. Can J Cardiol, 2010; 26(2): e50-53. doi: 10.1016/S0828-282X(10)70007-6 [99] de Servi S, Mussini A, Angoli L, et al. Effects of cold stimulation on coronary haemodynamics during exercise in patients with coronary artery disease. Eur Heart J, 1985; 6(3): 239-246. doi: 10.1093/oxfordjournals.eurheartj.a061847 [100] Peart I, Bullock R E, Albers C, et al. Cold intolerance in patients with angina pectoris: effect of nifedipine and propranolol. Br Heart J, 1989; 61(6): 521-528. doi: 10.1136/hrt.61.6.521 [101] Blanchet M, Ducharme A, Racine N, et al. Effects of cold exposure on submaximal exercise performance and adrenergic activation in patients with congestive heart failure and the effects of beta-adrenergic blockade (carvedilol or metoprolol). The Am J Cardiol, 2003; 92(5): 548-553. doi: 10.1016/S0002-9149(03)00723-9 [102] Juneau M, Larivée L, White M. Cold temperature impairs maximal exercise performance in patients with heart failure: attenuation by acute ACE inhibitor therapy. Can J Cardiol, 2002; 18(9): 981-986. [103] Schmid J P, Morger C, Noveanu M, et al. Haemodynamic and arrhythmic effects of moderately cold (22 degrees C) water immersion and swimming in patients with stable coronary artery disease and heart failure. Eur J Heart Fail, 2009; 11(9): 903-909. doi: 10.1093/eurjhf/hfp114 [104] Muller M D, Gunstad J, Alosco M L, et al. Acute cold exposure and cognitive function: evidence for sustained impairment. Ergonomics, 2012; 55(7): 792-798. doi: 10.1080/00140139.2012.665497 [105] Nakajima Y, Schmidt S M, Malmgren F A, et al. Relationship between Perceived Indoor Temperature and Self-Reported Risk for Frailty among Community-Dwelling Older People. Int J Environ Res Public Health, 2019; 16(4): 613. doi: 10.3390/ijerph16040613 [106] Manhães de Castro R, Bolaños-Jiménez F, Seguin L, et al. Subchronic cold stress reduces 5-HT1A receptor responsiveness in the old but not in the young rat. Neurosci Lett, 1996; 203(1): 21-24. doi: 10.1016/0304-3940(95)12253-2 [107] Steenman M, Lande G. Cardiac aging and heart disease in humans. Biophys Rev, 2017; 9(2): 131-137. doi: 10.1007/s12551-017-0255-9 -


 投稿系统
投稿系统


 下载:
下载: