Traditional Chinese medicine in the treatment of high incidence diseases in cold areas: the thrombotic diseases
doi: 10.2478/fzm-2021-0005
-
Abstract: Thrombotic diseases are the leading causes of death worldwide, especially in cold climates. Traditional Chinese medicine (TCM)-based therapies have gained increasing popularity worldwide, but also raised some concerns about its efficacy, safety profile and exact mechanisms. TCM has been traditionally used in the management of thrombosis and convincingly proven effective in modifying thrombosis progression, particularly the platelet function, coagulation system and fibrinolytic system. This review article focuses on TCM regulation of thrombosis with brief discussion on the fundamental aspects and relevant background information for better understanding of the subject. In addition to its antithrombotic effects, we will dive insight into the cellular and molecular mechanisms of TCM as pharmacological regulators of platelet aggregation, coagulation, and fibrinolysis. With increasing awareness and understanding of the benefits and potentials of TCM, TCM products will in no doubt gain its broader applications in the treatment of thrombosis and associated disorders, which in turn will deepen our understanding of its pharmacological and molecular mechanisms. Finally, current review provides a perspective view on the future directions to TCM research on thrombosis.
-
Key words:
- thrombosis /
- Traditional Chinese Medicine /
- platelet /
- coagulation /
- fibrinolysis
-
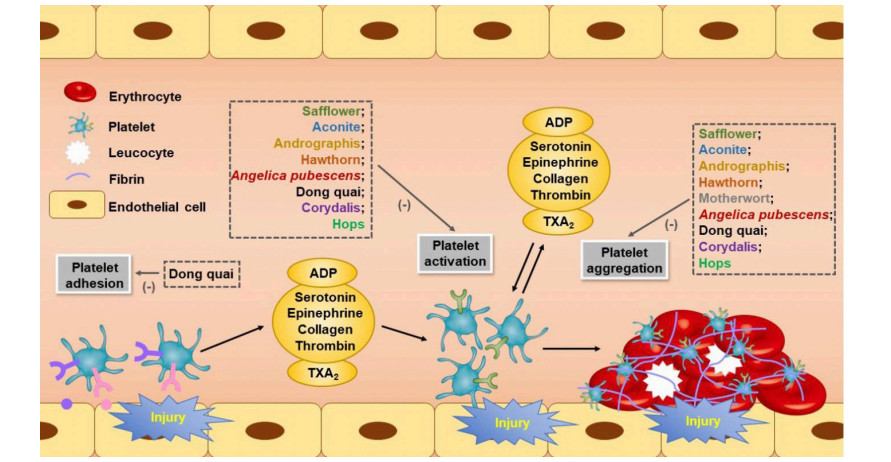
Table 1. Summary of TCMs and their effects on thrombosis.
Common name Latin name Active compounds Mechanism Reference Safflower Carthamus tinctorius SY, HSYA Reduced platelet aggregation; inhibited PAF receptor; inhibited serotonin release; decreased intraplatelet Ca2+; inhibited TXA2 production; reduced GP IIb/IIIa expression 25-30 Aconite Radix Aconiti praeparata Higenamine Reduced platelet aggregation; inhibited α2-adrenergic receptor; inhibited TXA2 receptor 32-34 Andrographis Andrographis paniculata AP1, AP3 Reduced platelet aggregation; decreased intraplatelet Ca2+; increase cGMP levels 38-40 Hawthorn Crataegus pinnatifida Eriodectyol Reduced platelet aggregation; inhibited TXA2 production; inhibited serotonin release 42-45 Motherwort Leonurus japonicus. Spirolabdane diterpenoids Reduced platelet aggregation 47-50 - Angelica pubescens Coumarin Reduced platelet aggregation; inhibited 5-LOX and COX-1 activity 52-54 Dong quai Angelica sinensis SF, LIG Reduced platelet aggregation; inhibited TXA2 and TXB2 production; suppressed 6-keto PGF1α generation; reduced vWF antigen and α-granule membrane protein 55-63 Corydalis Corydalis yanhusuo DHC, THB Reduced platelet aggregation; impaired thromboxane formation and phosphoinositides breakdown; decreased intraplatelet Ca2+; bound to ADP receptors P2Y1 and P2Y12, thrombin receptor PAR1 65-67 Hops Humulus lupulus L. XN Reduced platelet aggregation; inhibited TXA2 production; decreased intraplatelet Ca2+; decreased ROS accumulation and platelet mtDNA release 69-71 Liquorice Glycyrrhiza glabra Glycyrrhetinic acid, GL Inhibited thrombin; inhibited FXa 78-80 Feces Trogopterus Kaempferol coumaroyl rhamnosides Inhibited thrombin 83, 84 - Zanthoxylum nitidum var. tomentosum Toddalolactone Inhibited PAI-1 92 Pillbug Porcellio scaber Latreille PSLTro01 Activated plasminogen 94 Earthworm Eisenia fetida/Lumbricus rubellus EfP, LrP Activated plasminogen; directly degraded fibrin 95-101 Ginkgo Ginkgo biloba L. Ginkgolides, BB Reduced platelet aggregation; inhibited PAF receptor and glycine receptor; decreased intraplatelet Ca2+; inhibited TXA2 production; increase cAMP and cGMP levels 103-106 Ginkgetin, isoginkgetin, bilobetin, amentoflavone Inhibited thrombin 107 EGb761 Increased TM and t-PA expression and activity 108, 109 Asian Ginseng Panax ginseng Ginsenoside Rgl, Rp3, Ro, Rp1, gintonin Reduced platelet aggregation; decreased intraplatelet Ca2+; inhibited TXA2 production; inhibited serotonin release; inhibited ATP release; impaired GPVI signaling 111-116 Ginsenosides Rg2, Rg3 Inhibited FXa 117 Ginsenosides Rg1 Increased plasminogen activator secretion 118 Notoginseng Panax notoginseng Ginsenoside Rg1, notoginsenoside Fc Reduced platelet aggregation 119-122 Notoginsenoside R1 Increased t-PA and u-PA expression; decreased PAI-1 activity 123 Danshen Salvia miltiorrhiza Bge. SA Reduced platelet aggregation; decreased TXB2 and vWF levels; increased 6-keto-PGF1α levels; increased cAMP level 125-127 Tanshinones Enhanced AT-III and PC activities; inhibited FXa; inhibited thrombin; inhibited FVIIa 127-129 - Decreased plasma PAI-1 levels; increased t-PA levels 127 - Coptis chinensis BBR Reduced platelet aggregation; inhibited TXA2 production; inhibited α2-adrenergic receptor 132, 133 BBR Inhibited thrombin 134 Chuanxiong Ligusticum wallichii Tetramethylpyra-zine, SF Reduced platelet aggregation; inhibited TXA2 production; reduced GP IIb/IIIa expression; reduced NO production; increased cAMP level 136-139 - Inhibited thrombin 140 Ginger Zingiber officinale Gingerol, zingerone Reduced platelet aggregation; inhibited LOX and COX activity; inhibited TXA2 production; decreased intraplatelet Ca2+; reduced P-selectin and PAC-1 expression 142-146 Zingerone Inhibited FXa production and activity 146 - Celastrus orbiculatus NST-50 Reduced platelet aggregation; inhibited TXB2 production; increased 6-keto PGF1α generation 149 NST-50 Decreased PAI-1 levels; increased t-PA levels 149 Baical skullcap root Scutellaria baicalensis Georgi OroA, baicalin, WGN, WGNS Reduced platelet aggregation 151-154 OroA, baicalin, WGN, WGNS Inhibited thrombin production and activity; inhibited FXa production and activity; inhibited vitamin K reductases; inhibited TF production and activity 152-156 OroA, baicalin, WGN, Decreased PAI-1 levels WGNS 152-154 - Dioscorea zingiberensis TSSN, diosgenin Reduced platelet aggregation 158-160 - Inhibited FVIII 160 Madder Rubia cordifolia - Reduced platelet aggregation; inhibited TXB2 production; increased 6-keto PGF1α generation 162 Purpurin Inhibited FVII 163 Purpurin Increased t-PA levels; activated pro-urokinase 162, 163 Astragalus Astragalus membranaceus AGS-IV Bound to prothrombin and AT-III 165 AGS-IV Decreased PAI-1 levels; increased t-PA levels 166 Peony root Paeoniae lactiflora Paeoniflorin Reduced platelet aggregation; inhibited TXA2 and TXB2 production; increased 6-keto PGF1α generation; modulated vWF-GP Ib interaction 167-170 Paeoniflorin Increased t-PA activity; increased u-PA levels 169, 171 Turmeric Curcuma Longa Curcumin, curdione Reduced platelet aggregation; inhibited TXB2 production; decreased intraplatelet Ca2+; inhibited dense granule secretion; inhibited P-selectin expression; increased cAMP levels 173-178 Curcumin Inhibited thrombin; inhibited FXa; inhibited FVII synthesis 179, 180 Curcumin Increased u-PA levels 181 Yellow mealworm Tenebrio molitor - Reduced platelet aggregation; decreased intraplatelet Ca2+; inhibited P-selectin and PAC-1 expression; improved NO production; inhibited ET-1 secretion 183 - Inhibited FXa production and activity 183 safflower yellow; HSYA: hydroxysafflor yellow A; AP1: andrographolide; AP3: 14-deoxy-11, 12 didehydroandrographolide; PAF: platelet activating factor; TXA2: thromboxane A2; GP: glycoprotein; cGMP: cyclic guanosine monophosphate; LOX: lipoxygenase; COX: cyclooxygenase; SF: sodium ferulate; LIG: Z-ligustilide; TXB2: thromboxane B2; PG: prostaglandins; vWF: von Willebrand Factor; DHC: dehydrocorydaline; THB: canadine; ADP: adenosine diphosphate; ATP: adenosine triphosphate; XN: xanthohumol; GL: glycyrrhizin; PAI-1: plasminogen activator inhibitor 1; EfP: E. fetida proteases; LrP: L. rubellus proteases; BB: bilobalide; TM: thrombomodulin; t-PA: tissuetype plasminogen activator; u-PA: urokinasetype plasminogen activator; SA: salvianolic acid; BBR: berberine; OroA: oroxylin A; WGN: wogonin; WGNS: wogonoside; TSSN: total steroidal saponin; AGS-IV: astragaloside IV; cAMP: cyclic adenosine monophosphate; AT-III: antithrombin III; PC: protein C; ET-1: endothelin-1 -
[1] Koupenova M, Kehrel B E, Corkrey H A, et al. Thrombosis and platelets: an update. Eur Heart J, 2017; 38(11): 785-791. [2] Bhaskaran K, Hajat S, Haines A, et al. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ, 2010; 341: c3823. doi: 10.1136/bmj.c3823 [3] Pant S, Badheka A O, Deshmukh A. Higher frequency of atrial fibrillation linked to colder seasons and air temperature on the day of ischemic stroke onset. J Stroke Cerebrovasc Dis, 2013; 22: 896. http://europepmc.org/abstract/med/23562211 [4] Awad E M, Khan S Y, Sokolikova B, et al. Cold induces reactive oxygen species production and activation of the NF-kappa B response in endothelial cells and inflammation in vivo. J Thromb Haemost, 2013; 11(9): 1716-1726. doi: 10.1111/jth.12357 [5] Rosendaal F R. Risk factors for venous thrombotic disease. Thromb Haemost, 1999; 82(9): 610-619. http://www.onacademic.com/detail/journal_1000040892555010_4523.html [6] Birk S, Kruuse C, Petersen K A, et al. The headache-inducing effect of cilostazol in human volunteers. Cephalalgia, 2006; 26(11): 1304-1309. doi: 10.1111/j.1468-2982.2006.01218.x [7] Francescone S, Halperin J L. "Triple therapy" or triple threat? Balancing the risks of antithrombotic therapy for patients with atrial fibrillation and coronary stents. J Am Coll Cardiol, 2008; 51(8): 826-827. doi: 10.1016/j.jacc.2007.11.034 [8] Johnson S. Known knowns and known unknowns: risks associated with combination antithrombotic therapy. Thromb Res, 2008; 123(Supp l 1): S7-S11. http://www.onacademic.com/detail/journal_1000035441286010_a236.html [9] Alban S. Adverse effects of heparin. Handb Exp Pharmacol, 2012: 211-263. http://europepmc.org/abstract/MED/22566227 [10] Flemmig M, Melzig M F. Serine-proteases as plasminogen activators in terms of fibrinolysis. J Pharm Pharmacol, 2012; 64(8): 1025-1039. doi: 10.1111/j.2042-7158.2012.01457.x [11] Disharoon D, Marr D W M, Neeves K B. Engineered microparticles and nanoparticles for fibrinolysis. J Thromb Haemost, 2019; 17(12): 2004-2015. doi: 10.1111/jth.14637 [12] Lu A P, Jia H W, Xiao C, et al. Theory of traditional Chinese medicine and therapeutic method of diseases. World J Gastroenterol, 2004; 10(13): 1854-1856. doi: 10.3748/wjg.v10.i13.1854 [13] Hsiao W L, Liu L. The role of traditional Chinese herbal medicines in cancer therapy--from TCM theory to mechanistic insights. Planta Med, 2010; 76(11): 1118-1131. doi: 10.1055/s-0030-1250186 [14] Yang R, Yuan B C, Ma YS, et al. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol, 2017; 55(1): 5-18. doi: 10.1080/13880209.2016.1225775 [15] Ma C, Xia R, Yang S, et al. Formononetin attenuates atherosclerosis via regulating interaction between KLF4 and SRA in apoE(-/-) mice. Theranostics, 2020; 10(3): 1090-1106. doi: 10.7150/thno.38115 [16] Zhai B, Zhang N, Han X, et al. Molecular targets of beta-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: A review. Biomed Pharmacother, 2019; 114: 108812. doi: 10.1016/j.biopha.2019.108812 [17] Bai L, Li X, He L, et al. Antidiabetic potential of flavonoids from traditional Chinese medicine: a review. Am J Chin Med, 2019; 47(5): 933-957. doi: 10.1142/S0192415X19500496 [18] Ren J L, Zhang A H, Wang X J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res, 2020; 155(8): 104743. http://qikan.cqvip.com/Qikan/Article/Detail?id=00004GOIK77XLEODLLEFKJH1MLDO8IP1MPD1Q [19] Li Y, Liu X, Guo L, et al. Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: protocol for a systematic review and meta-analysis. Syst Rev, 2020; 9(1): 75. doi: 10.1186/s13643-020-01343-4 [20] Holinstat M. Normal platelet function. Cancer Metastasis Rev, 2017; 36(2): 195-198. doi: 10.1007/s10555-017-9677-x [21] Stalker T J, Traxler E A, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood, 2013; 121(10): 1875-1885. doi: 10.1182/blood-2012-09-457739 [22] Welsh J D, Muthard R W, Stalker T J, et al. A systems approach to hemostasis: 4. How hemostatic thrombi limit the loss of plasma-borne molecules from the microvasculature. Blood, 2016; 127(12): 1598-1605. doi: 10.1182/blood-2015-09-672188 [23] Delshad E, Yousefi M, Sasannezhad P, et al. Medical uses of carthamus tinctorius L. (Safflower): a comprehensive review from traditional medicine to modern medicine. Electron Physician, 2018; 10(4): 6672-6681. doi: 10.19082/6672 [24] Chinese Center for Disease Control and Prevention. Guide Manual on Pharmacological Management of Coronavirus Diseases 2019(COVID-19). Beijing: People's Medical Publishing House, 2020. [25] Zhou X, Tang L, Xu Y, et al. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol, 2014; 151(1): 27-43. doi: 10.1016/j.jep.2013.10.050 [26] Memariani Z, Moeini R, Hamedi S S, et al. Medicinal plants with antithrombotic property in Persian medicine: a mechanistic review. J Thromb Thrombolysis, 2018; 45(1): 158-179. doi: 10.1007/s11239-017-1580-3 [27] Zang B X, Jin M, Si N, et al. Antagonistic effect of hydroxysafflor yellow A on the platelet activating factor receptor. Yao Xue Xue Bao, 2002; 37(9): 696-699. http://www.ncbi.nlm.nih.gov/pubmed/12567893 [28] Zhu H B, Zhang L, Wang Z H, et al. Therapeutic effects of hydroxysafflor yellow A on focal cerebral ischemic injury in rats and its primary mechanisms. J Asian Nat Prod Res, 2005; 7(4): 607-613. doi: 10.1080/10286020310001625120 [29] Li Y, Wang N. Antithrombotic effects of Danggui, Honghua and potential drug interaction with clopidogrel. J Ethnopharmacol, 2010; 128(3): 623-628. doi: 10.1016/j.jep.2010.02.003 [30] Zhu Y F, Luo H M, Deng Z L, et al. Effects of the Chinese patent medicine, Honghua Injection, on platelet glycoprotein Ⅱb/Ⅲ a receptors in patients with acute coronary syndrome: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao, 2012; 10(3): 318-323. doi: 10.3736/jcim20120311 [31] Zhang N, Lian Z, Peng X, et al. Applications of Higenamine in pharmacology and medicine. J Ethnopharmacol, 2017; 196(20): 242-252. http://or.nsfc.gov.cn/bitstream/00001903-5/495859/1/991482576.pdf [32] Yun-Choi H S, Pyo M K, Park K M, et al. Anti-thrombotic effects of higenamine. Planta Med 2001; 67(7): 619-622. doi: 10.1055/s-2001-17361 [33] Yun-Choi H S, Pyo M K, Park K M, et al. Antithrombotic effects of YS-49 and YS-51--1-naphthylmethyl analogs of higenamine. Thromb Res, 2001; 104(4): 249-255. doi: 10.1016/S0049-3848(01)00372-3 [34] Pyo M K, Kim J M, Jin J L, et al. Effects of higenamine and its 1-naphthyl analogs, YS-49 and YS-51, on platelet TXA2 synthesis and aggregation. Thromb res, 2007; 120(1): 81-86. doi: 10.1016/j.thromres.2006.07.006 [35] Chang R S, Ding L, Chen G Q, et al. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Proc Soc Exp Biol Med, 1991; 197(1): 59-66. doi: 10.3181/00379727-197-43225 [36] Calabrese C, Berman S H, Babish J G, et al. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res, 2000; 14(5): 333-338. doi: 10.1002/1099-1573(200008)14:5<333::AID-PTR584>3.0.CO;2-D [37] Shen Y C, Chen C F, Chiou W F. Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. Br J Pharmacol, 2002; 135(2): 399-406. doi: 10.1038/sj.bjp.0704493 [38] See D, Mason S, Roshan R. Increased tumor necrosis factor alpha (TNF-alpha) and natural killer cell (NK) function using an integrative approach in late stage cancers. Immunol Invest, 2002; 31: 137-153. doi: 10.1081/IMM-120004804 [39] Kapil A, Koul I B, Banerjee S K, et al. Antihepatotoxic effects of major diterpenoid constituents of Andrographis paniculata. Biochem Pharmacol, 1993; 46(1): 182-185. doi: 10.1016/0006-2952(93)90364-3 [40] Zhang X F, Tan B K. Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats. Clin Exp Pharmacol Physiol, 2000; 27(5-6): 358-363. doi: 10.1046/j.1440-1681.2000.03253.x [41] Dehghani S, Mehri S, Hosseinzadeh H. The effects of Crataegus pinnatifida (Chinese hawthorn) on metabolic syndrome: A review. Iran J Basic Med Sci, 2019; 22(5): 460-468. http://ijbms.mums.ac.ir/article_12571_365a4e3bccd663033089f420fc25ed34.pdf [42] Arslan R, Bor Z, Bektas N, et al. Antithrombotic effects of ethanol extract of Crataegus orientalis in the carrageenan-induced mice tail thrombosis model. Thromb Res, 2011; 127(3): 210-213. doi: 10.1016/j.thromres.2010.11.028 [43] Song S J, Li L Z, Gao P Y, et al. Isolation of antithrombotic phenolic compounds from the leaves of crataegus pinnatifida. Planta Med, 2012; 78(18): 1967-1971. doi: 10.1055/s-0032-1327877 [44] Rogers K L, Grice I D, Griffiths L R. Inhibition of platelet aggregation and 5-HT release by extracts of Australian plants used traditionally as headache treatments. Eur J Pharm Sci, 2000; 9(4): 355-363. doi: 10.1016/S0928-0987(99)00074-3 [45] Vibes J, Lasserre B, Gleye J, et al. Inhibition of thromboxane A2 biosynthesis in vitro by the main components of Crataegus oxyacantha (Hawthorn) flower heads. Prostaglandins Leukot Essent Fatty Acids, 1994; 50(4): 173-175. doi: 10.1016/0952-3278(94)90141-4 [46] Shang X, Pan H, Wang X, et al. Leonurus japonicus Houtt. : ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol, 2014; 152(1): 14-32. doi: 10.1016/j.jep.2013.12.052 [47] Moon H I. Three diterpenes from Leonurus japonicus Houtt protect primary cultured rat cortical cells from glutamate-induced toxicity. Phytother Res, 2010; 24(8): 1256-1259. doi: 10.1002/ptr.3144 [48] Xiong L, Zhou Q M, Peng C, et al. Bis-spirolabdane diterpenoids from Leonurus japonicus and their anti-platelet aggregative activity. Fitoterapia, 2015; 100(1): 1-6. http://www.onacademic.com/detail/journal_1000036990802310_62fd.html [49] Xiong L, Zhou Q M, Peng C, et al. Sesquiterpenoids from the herb of Leonurus japonicus. Molecules, 2013; 18(5): 5051-5058. doi: 10.3390/molecules18055051 [50] Zou Q Z, Bi R G, Li J M, et al. Effect of motherwort on blood hyperviscosity. Am J Chin Med, 1989; 17(1-2): 65-70. http://www.onacademic.com/detail/journal_1000037390685510_ca99.html [51] Chen D, Du Z, Lin Z, et al. The chemical compositions of angelica pubescens oil and its prevention of UV-B radiation-induced cutaneous photoaging. Chem Biodivers, 2018; 15(10): e1800235. doi: 10.1002/cbdv.201800235 [52] Liu J H, Zschocke S, Reininger E, et al. Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase. Planta Med, 1998; 64(6): 525-529. doi: 10.1055/s-2006-957507 [53] Hoult J R, Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol, 1996; 27(4): 713-722. doi: 10.1016/0306-3623(95)02112-4 [54] Hou S M, Hsia C W, Tsai C L, et al. Modulation of human platelet activation and in vivo vascular thrombosis by columbianadin: regulation by integrin alphaⅡbbeta3 inside-out but not outside-in signals. J Biomed Sci, 2020; 27(1): 60. doi: 10.1186/s12929-020-0619-5 [55] Wang B H, Ou-Yang J P. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc Drug Rev, 2005; 23(2): 161-172. http://www.nevapress.com/cdr/Abstracts/23/161a.pdf [56] Xu L N, Ouyang R. Antithrombotic effect of sodium ferulate in rats (author's transl)]. Zhongguo Yao Li Xue Bao, 1981; 2(1): 35-37. http://www.europepmc.org/abstract/med/6461198 [57] Yin Z Z, Zhang L Y, Xu L N. The effect of Dang-Gui (Angelica sinensis) and its ingredient ferulic acid on rat platelet aggregation and release of 5-HT (author's transl)]. Yao Xue Xue Bao, 1980; 15(6): 321-326. http://europepmc.org/abstract/MED/7457152 [58] Yin Z Z, Wang J P, Xu L N. Effect of sodium ferulate on malondialdehyde production from the platelets of rats. Zhongguo Yao Li Xue Bao, 1986; 7(4): 336-339. http://www.europepmc.org/abstract/med/2954394 [59] Wang Z, Gao Y H, Huang R S, et al. Sodium ferulate is an inhibitor of thromboxane A2 synthetase. Zhongguo Yao Li Xue Bao, 1988; 9(5): 430-433. http://www.chinaphar.com/article/view/8665/9627 [60] Xu L N, Yu W G, Tian JY. Effect of sodium ferulate on C14-arachidonic acid metabolism in rabbit platelets. Zhong Xi Yi Jie He Za Zhi, 1988; 8(10): 614-615, 583. http://www.ncbi.nlm.nih.gov/pubmed/3255545/ [61] Xu L N, Yu W G, Tian J Y, et al. Effect of sodium ferulate on arachidonic acid metabolism. Yao Xue Xue Bao, 1990; 25(6): 412-416. http://europepmc.org/abstract/med/2284965 [62] Zhang L, Du J R, Wang J, et al. Z-ligustilide extracted from Radix Angelica Sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi, 2009; 129(7): 855-859. doi: 10.1248/yakushi.129.855 [63] Dong W G, Liu S P, Zhu H H, et al. Abnormal function of platelets and role of angelica sinensis in patients with ulcerative colitis. World J Gastroenterol, 2004; 10(4): 606-609. doi: 10.3748/wjg.v10.i4.606 [64] Chang S, Yang Z, Han N, et al. The antithrombotic, anticoagulant activity and toxicity research of ambinine, an alkaloid from the tuber of Corydalis ambigua var. amurensis. Regul Toxicol Pharmacol, 2018; 95: 175-181. doi: 10.1016/j.yrtph.2018.03.004 [65] Ko F N, Wu T S, Lu S T, et al. Antiplatelet effects of protopine isolated from Corydalis tubers. Thromb Res, 1989; 56(2): 289-298. doi: 10.1016/0049-3848(89)90170-9 [66] Tan C N, Zhang Q, Li C H, et al. Potential target-related proteins in rabbit platelets treated with active monomers dehydrocorydaline and canadine from Rhizoma corydalis. Phytomedicine, 2019; 54: 231-239. doi: 10.1016/j.phymed.2018.09.200 [67] Li C H, Chen C, Zhang Q, et al. Differential proteomic analysis of platelets suggested target-related proteins in rabbit platelets treated with Rhizoma Corydalis. Pharm Biol, 2017; 55(1): 76-87. doi: 10.1080/13880209.2016.1229340 [68] Zanoli P, Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J Ethnopharmacol, 2008; 116(3): 383-396. doi: 10.1016/j.jep.2008.01.011 [69] Xin G, Wei Z, Ji C, et al. Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release. Free Radic Biol Med, 2017; 108: 247-257. doi: 10.1016/j.freeradbiomed.2017.02.018 [70] Luzak B, Kassassir H, Roj E, et al. Xanthohumol from hop cones (Humulus lupulus L. ) prevents ADP-induced platelet reactivity. Arch Physiol Biochem, 2017; 123(1): 54-60. doi: 10.1080/13813455.2016.1247284 [71] Lee Y M, Hsieh K H, Lu W J, et al. Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus), Prevents Platelet Activation in Human Platelets. Evid Based Complement Alternat Med, 2012: 852362. http://www.researchgate.net/profile/Hsiu-Chu_Chou/publication/225050797_Xanthohumol_a_Prenylated_Flavonoid_from_Hops_(Humulus_lupulus)_Prevents_Platelet_Activation_in_Human_Platelets/links/02e7e52af96bc2da69000000.pdf [72] Adams R L, Bird R J. Review article: Coagulation cascade and therapeutics update: relevance to nephrology. Part 1: Overview of coagulation, thrombophilias and history of anticoagulants. Nephrology (Carlton), 2009; 14(5): 462-470. doi: 10.1111/j.1440-1797.2009.01128.x [73] Tanaka K A, Key N S, Levy J H. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg, 2009; 108(5): 1433-1446. doi: 10.1213/ane.0b013e31819bcc9c [74] Versteeg H H, Heemskerk J W, Levi M, et al. New fundamentals in hemostasis. Physiol Rev, 2013; 93(1): 327-358. doi: 10.1152/physrev.00016.2011 [75] Ten Cate H, Hackeng T M, Garcia de Frutos P. Coagulation factor and protease pathways in thrombosis and cardiovascular disease. Thromb Haemost, 2017; 117(7): 1265-1271. doi: 10.1160/TH17-02-0079 [76] Gelosa P, Castiglioni L, Tenconi M, et al. Pharmacokinetic drug interactions of the non-vitamin K antagonist oral anticoagulants (NOACs). Pharmacol Res, 2018; 135: 60-79. doi: 10.1016/j.phrs.2018.07.016 [77] Pastorino G, Cornara L, Soares S, et al. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res, 2018; 32(3): 2323-2339. doi: 10.1002/ptr.6178 [78] Francischetti I M, Monteiro R Q, Guimaraes J A. Identification of glycyrrhizin as a thrombin inhibitor. Biochem Biophys Res Commun, 1997; 235(1): 259-263. doi: 10.1006/bbrc.1997.6735 [79] Mendes-Silva W, Assafim M, Ruta B, et al. Antithrombotic effect of Glycyrrhizin, a plant-derived thrombin inhibitor. Thromb res, 2003; 112(1-2): 93-98. doi: 10.1016/j.thromres.2003.10.014 [80] Jiang L, Wang Q, Shen S, et al. Discovery of glycyrrhetinic acid as an orally active, direct inhibitor of blood coagulation factor xa. Thromb Res, 2014; 133(3): 501-506. doi: 10.1016/j.thromres.2013.12.025 [81] Xiao X, Zhou S H, Jiang N, et al. First record of Leptospira and Blastocystis infections in captive flying squirrels (Trogopterus xanthipes) from Enshi County, China. Acta Trop, 2019; 197: 105065. doi: 10.1016/j.actatropica.2019.105065 [82] Baek S, Xia X, Min B S, et al. Trogopterins A-C: Three new neolignans from feces of Trogopterus xanthipes. Beilstein J Org Chem, 2014; 10: 2955-2962. doi: 10.3762/bjoc.10.313 [83] Yang N Y, Tao W W, Duan J A. Antithrombotic flavonoids from the faeces of Trogopterus xanthipes. Nat Prod Res, 2010; 24: 1843-1849. doi: 10.1080/14786419.2010.482057 [84] Yang N Y, Tao W W, Duan J A, et al. Four new fatty acid esters from the Feces of Trogopterus xanthipes. Lipids, 2009; 44(9): 849-853. doi: 10.1007/s11745-009-3329-z [85] Kumar S S, Sabu A. Fibrinolytic enzymes for thrombolytic therapy. Adv Exp Med Biol, 2019; 1148: 345-381. http://www.ncbi.nlm.nih.gov/pubmed/31482506/ [86] Rabinstein A A. Treatment of Acute Ischemic Stroke. Continuum (Minneap Minn), 2017; 23(1): 62-81. http://pdfs.semanticscholar.org/7d97/ebfef6405f6af545dc44b3f11d0a9837dce4.pdf [87] Gurewich V. Fibrinolysis: a misunderstood natural defense whose therapeutic potential is unknown. Cardiovasc Drugs Ther, 2019; 33: 749-753. doi: 10.1007/s10557-019-06923-8 [88] Liesenborghs L, Verhamme P, Vanassche T. Staphylococcus aureus, master manipulator of the human hemostatic system. J Thromb Haemost, 2018; 16: 441-454. doi: 10.1111/jth.13928 [89] Marder V J. Historical perspective and future direction of thrombolysis research: the re-discovery of plasmin. J Thromb Haemost, 2011; 9(Suppl 1): 364-373. doi: 10.1111/j.1538-7836.2011.04370.x/pdf [90] Emberson J, Lees K R, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet, 2014; 384(9958): 1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [91] Lu Q, Ma R, Yang Y, et al. Zanthoxylum nitidum (Roxb. ) DC: Traditional uses, phytochemistry, pharmacological activities and toxicology. J Ethnopharmacol, 2020; 260(5): 112946. http://www.ncbi.nlm.nih.gov/pubmed/32492492 [92] Yu B, Zhang G, Jin L, et al. Inhibition of PAI-1 activity by toddalolactone as a mechanism for promoting blood circulation and removing stasis by Chinese herb zanthoxylum nitidum var. tomentosum. Front Pharmacol, 2017; 8: 489. doi: 10.3389/fphar.2017.00489 [93] Huber J, Fabritius H O, Griesshaber E, et al. Function-related adaptations of ultrastructure, mineral phase distribution and mechanical properties in the incisive cuticle of mandibles of porcellio scaber latreille, 1804. J Struct Biol, 2014; 188(1): 1-15. doi: 10.1016/j.jsb.2014.09.002 [94] Tian Z, Li B, Guo L, et al. Purification and biochemical characterization of a novel fibrinolytic enzyme, PSLTro01, from a medicinal animal Porcellio scaber Latreille. Int J Biol Macromol, 2015; 80: 536-546. doi: 10.1016/j.ijbiomac.2015.06.046 [95] Nakajima N, Ishihara K, Sugimoto M, et al. Chemical modification of earthworm fibrinolytic enzyme with human serum albumin fragment and characterization of the protease as a therapeutic enzyme. Biosci Biotechnol Biochem, 1996; 60(2): 293-300. doi: 10.1271/bbb.60.293 [96] Wang F, Wang C, Li M, et al. Purification, characterization and crystallization of a group of earthworm fibrinolytic enzymes from Eisenia fetida. Biotechnol Lett, 2003; 25: 1105-1109. doi: 10.1023/A:1024196232252 [97] Cho I H, Choi E S, Lim H G, et al. Purification and characterization of six fibrinolytic serine-proteases from earthworm Lumbricus rubellus. J Biochem Mol Biol, 2004; 37(2): 199-205. http://www.jbmb.or.kr/jbmb/jbmb_files/[37-2]0404011548_199-205.pdf [98] Lee C K, Shin J S, Kim B S, et al. Antithrombotic effects by oral administration of novel proteinase fraction from earthworm Eisenia andrei on venous thrombosis model in rats. Arch Pharm Res, 2007; 30(4): 475-480. doi: 10.1007/BF02980222 [99] Tjandrawinata R R, Yunaidi D A, Susanto L W. The safety and tolerability of lumbrokinase DLBS1033 in healthy adult subjects. Drug Res (Stuttg), 2016; 66(6): 293-299. doi: 10.1055/s-0035-1569406 [100] Fan Q, Wu C, Li L, et al. Some features of intestinal absorption of intact fibrinolytic enzyme Ⅲ-1 from Lumbricus rubellus. Biochim Biophys Acta, 2001; 1526(3): 286-292. doi: 10.1016/S0304-4165(01)00140-4 [101] Nakajima N, Sugimoto M, Ishihara K. Stable earthworm serine proteases: application of the protease function and usefulness of the earthworm autolysate. J Biosci Bioeng, 2000; 90(2): 174-179. doi: 10.1016/S1389-1723(00)80106-1 [102] Mahady G B. Ginkgo biloba for the prevention and treatment of cardiovascular disease: a review of the literature. J Cardiovasc Nurs, 2002; 16(4): 21-32. doi: 10.1097/00005082-200207000-00004 [103] Stromgaard K, Nakanishi K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angew Chem Int Ed Engl, 2004; 43(13): 1640-1658. doi: 10.1002/anie.200300601 [104] Nakanishi K. Terpene trilactones from Gingko biloba: from ancient times to the 21st century. Bioorg Med Chem, 2005; 13(17): 4987-5000. doi: 10.1016/j.bmc.2005.06.014 [105] Cho H J, Nam K S. Inhibitory effect of ginkgolide B on platelet aggregation in a cAMP- and cGMP-dependent manner by activated MMP-9. J Biochem Mol Biol, 2007; 40: 678-683. http://www.jbmb.or.kr/jbmb/jbmb_files/[40-5]0710041348_678.pdf [106] Li X Y, Li C, Liu T, et al. Chemical analysis, pharmacological activity and process optimization of the proportion of bilobalide and ginkgolides in Ginkgo biloba extract. J Pharm Biomed Anal, 2018; 160: 46-54. doi: 10.1016/j.jpba.2018.07.037 [107] Chen T R, Wei L H, Guan X Q, et al. Biflavones from Ginkgo biloba as inhibitors of human thrombin. Bioorg Chem, 2019; 92(Suppl): 103199. http://www.sciencedirect.com/science/article/pii/S0045206819300628 [108] Naderi G A, Asgary S, Jafarian A, et al. Fibrinolytic effects of Ginkgo biloba extract. Exp Clin Cardiol, 2005; 10(2): 85-87. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=PMC2716226&blobtype=pdf [109] Chiu Y L, Tsai W C, Wu C H, et al. Ginkgo biloba induces thrombomodulin expression and tissue-type plasminogen activator secretion via the activation of kruppel-like factor 2 within endothelial cells. Am J Chin Med, 2020; 48(2): 357-372. doi: 10.1142/S0192415X20500184 [110] Lee C H, Kim J H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res, 2014; 38(3): 161-166. doi: 10.1016/j.jgr.2014.03.001 [111] Jin Y R, Yu J Y, Lee J J, et al. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol, 2007; 100(3): 170-175. doi: 10.1111/j.1742-7843.2006.00033.x [112] Kimura Y, Okuda H, Arichi S. Effects of various ginseng saponins on 5-hydroxytryptamine release and aggregation in human platelets. J Pharm Pharmacol, 1988; 40(12): 838-843. http://www.ncbi.nlm.nih.gov/pubmed/2907575 [113] Irfan M, Jeong D, Kwon H W, et al. Ginsenoside-Rp3 inhibits platelet activation and thrombus formation by regulating MAPK and cyclic nucleotide signaling. Vascul Pharmacol, 2018; 109: 45-55. doi: 10.1016/j.vph.2018.06.002 [114] Shin J H, Kwon H W, Rhee M H, et al. Inhibitory effects of thromboxane A2 generation by ginsenoside Ro due to attenuation of cytosolic phospholipase A2 phosphorylation and arachidonic acid release. J Ginseng Res, 2019; 43(2): 236-241. doi: 10.1016/j.jgr.2017.12.007 [115] Endale M, Lee W M, Kamruzzaman S M, et al. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br J Pharmacol, 2012; 167(1): 109-127. doi: 10.1111/j.1476-5381.2012.01967.x [116] Irfan M, Jeong D, Saba E, et al. Gintonin modulates platelet function and inhibits thrombus formation via impaired glycoprotein VI signaling. Platelets, 2019; 30: 589-598. doi: 10.1080/09537104.2018.1479033 [117] Xiong L, Qi Z, Zheng B, et al. Inhibitory Effect of Triterpenoids from Panax ginseng on Coagulation Factor X. Molecules, 2017; 22: 649. doi: 10.3390/molecules22040649 [118] Ushio Y. Effect of ginsenoside Rgl on the release of enzymes by cultured endothelial cells. Am J Chin Med, 1992; 20(1): 91-101. doi: 10.1142/S0192415X92000102 [119] Lau A J, Toh D F, Chua T K, et al. Antiplatelet and anticoagulant effects of Panax notoginseng: comparison of raw and steamed Panax notoginseng with Panax ginseng and Panax quinquefolium. J Ethnopharmacol, 2009; 125(3): 380-386. doi: 10.1016/j.jep.2009.07.038 [120] Shen Q, Li J, Zhang C, et al. Panax notoginseng saponins reduce high-risk factors for thrombosis through peroxisome proliferator-activated receptor -gamma pathway. Biomed Pharmacother, 2017; 96: 1163-1169. doi: 10.1016/j.biopha.2017.11.106 [121] Zhou Q, Jiang L, Xu C, et al. Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thromb Res, 2014; 133(1): 57-65. doi: 10.1016/j.thromres.2013.10.032 [122] Liu Y, Liu T, Ding K, et al. Phospholipase cgamma2 signaling cascade contribute to the antiplatelet effect of notoginsenoside fc. Front Pharmacol, 2018; 9: 1293. doi: 10.3389/fphar.2018.01293 [123] Zhang W J, Wojta J, Binder B R. Effect of notoginsenoside R1 on the synthesis of components of the fibrinolytic system in cultured smooth muscle cells of human pulmonary artery. Cell mol biol (Noisy-le-grand), 1997; 43(4): 581-587. http://www.researchgate.net/profile/Johann_Wojta/publication/13999718_Effect_of_notoginsenoside_R1_on_the_synthesis_of_components_of_the_fibrinolytic_system_in_cultured_smooth_muscle_cells_of_human_pulmonary_artery/links/0912f50fff18ce11c7000000.pdf [124] Li Z M, Xu S W, Liu P Q. Salvia miltiorrhizaBurge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol Sin, 2018; 39(5): 802-824. doi: 10.1038/aps.2017.193 [125] Tang M K, Ren D C, Zhang J T, et al. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine, 2002; 9(5): 405-409. doi: 10.1078/09447110260571634 [126] Fan H Y, Fu F H, Yang M Y, et al. Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res, 2010; 126(1): e17-22. doi: 10.1016/j.thromres.2010.04.006 [127] Kasimu R, Wang X, Wang X, et al. Antithrombotic effects and related mechanisms of Salvia deserta Schang root EtOAc extracts. Sci Rep, 2018; 8(1): 17753. doi: 10.1038/s41598-018-36026-7 [128] Yang Y Y, Wu Z Y, Zhang H, et al. LC-MS-based multivariate statistical analysis for the screening of potential thrombin/factor Xa inhibitors from Radix Salvia Miltiorrhiza. Chin Med, 2020; 15(1): 38. doi: 10.1186/s13020-020-00320-2 [129] Wang D, Girard T J, Kasten T P, et al. Inhibitory activity of unsaturated fatty acids and anacardic acids toward soluble tissue factor-factor VⅡa complex. J Nat Prod, 1998; 61(11): 1352-1355. doi: 10.1021/np980117p [130] Wu J, Luo Y, Deng D, et al. Coptisine from Coptis chinensis exerts diverse beneficial properties: A concise review. J Cell Mol Med, 2019; 23: 7946-7960. doi: 10.1111/jcmm.14725 [131] Yang J, Ljn J. Advance on study in anti-tumor mechamism of bererine (Ber). Zhongguo Zhong Yao Za Zhi, 2007; 32(10): 881-883, 934. http://www.medicinabiomolecular.com.br/biblioteca/pdfs/Cancer/ca-2070.pdf [132] Huang C G, Chu Z L, Wei S J, et al. Effect of berberine on arachidonic acid metabolism in rabbit platelets and endothelial cells. Thromb Res, 2002; 106(4-5): 223-227. doi: 10.1016/S0049-3848(02)00133-0 [133] Hui K K, Yu J L, Chan W F, et al. Interaction of berberine with human platelet alpha 2 adrenoceptors. Life Sci, 1991; 49(4): 315-324. doi: 10.1016/0024-3205(91)90019-8 [134] Wang X, Zhang Y, Yang Y, et al. Identification of berberine as a direct thrombin inhibitor from traditional Chinese medicine through structural, functional and binding studies. Sci Rep, 2017; 7(1): 44040. doi: 10.1038/srep44040 [135] Ho J W, Jie M. Pharmacological activity of cardiovascular agents from herbal medicine. Cardiovasc Hematol Agents Med Chem, 2007; 5(4): 273-277. doi: 10.2174/187152507782109854 [136] Sheu J R, Kan Y C, Hung W C, et al. Mechanisms involved in the antiplatelet activity of tetramethylpyrazine in human platelets. Thromb Res, 1997; 88(3): 259-270. doi: 10.1016/S0049-3848(97)00253-3 [137] Liu S Y, Sylvester D M. Antiplatelet activity of tetramethylpyrazine. Thromb Res, 1994; 75: 51-62. doi: 10.1016/0049-3848(94)90139-2 [138] Sheu J R, Kan Y C, Hung W C, et al. The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase. Life Sci, 2000; 67: 937-947. doi: 10.1016/S0024-3205(00)00686-X [139] Li L, Chen H, Shen A, et al. Ligustrazine inhibits platelet activation via suppression of the Akt pathway. Int J Mol Med, 2019; 43(1): 575-582. http://www.ncbi.nlm.nih.gov/pubmed/30387814 [140] Zhang Q, Yang Y X, Li S Y, et al. An ultrafiltration and high performance liquid chromatography coupled with diode array detector and mass spectrometry approach for screening and characterizing thrombin inhibitors from Rhizoma Chuanxiong. J Chromatogr B Analyt Technol Biomed Life Sci, 2017; 1061-1062: 421-429. doi: 10.1016/j.jchromb.2017.07.050 [141] Tsui P F, Lin C S, Ho L J, et al. Spices and Atherosclerosis. Nutrients, 2018; 10(11): 1724. doi: 10.3390/nu10111724 [142] Srivas K C. Effects of aqueous extracts of onion, garlic and ginger on platelet aggregation and metabolism of arachidonic acid in the blood vascular system: in vitro study. Prostaglandins Leukot Med, 1984; 13(2): 227-235. doi: 10.1016/0262-1746(84)90014-3 [143] Srivastava K C. Effect of onion and ginger consumption on platelet thromboxane production in humans. Prostaglandins Leukot Essent Fatty Acids, 1989; 35(3): 183-185. doi: 10.1016/0952-3278(89)90122-1 [144] Koo K L, Ammit A J, Tran V H, et al. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb Res, 2001; 103(5): 387-397. doi: 10.1016/S0049-3848(01)00338-3 [145] Nurtjahja-Tjendraputra E, Ammit A J, Roufogalis B D, et al. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res, 2003; 111(4-5): 259-265. doi: 10.1016/j.thromres.2003.09.009 [146] Lee W, Ku S K, Kim M A, et al. Anti-factor Xa activities of zingerone with anti-platelet aggregation activity. Food Chem Toxicol, 2017; 105: 186-193. doi: 10.1016/j.fct.2017.04.012 [147] Jiang W, Shan T Z, Xu J J, et al. Cytotoxic abietane and kaurane diterpenoids from Celastrus orbiculatus. J Nat Med, 2019; 73(11): 841-846. doi: 10.1007/s11418-019-01326-3 [148] Guo Y Q, Li X, Xu J, et al. Sesquiterpene esters from the fruits of Celastrus orbiculatus. Chem Pharm Bull (Tokyo), 2004; 52(9): 1134-1136. doi: 10.1248/cpb.52.1134 [149] Zhou J, Zhai J, Zheng W, et al. The antithrombotic activity of the active fractions from the fruits of Celastrus orbiculatus Thunb through the anti-coagulation, anti-platelet activation and anti-fibrinolysis pathways. J Ethnopharmacol, 2019; 241: 111974. doi: 10.1016/j.jep.2019.111974 [150] Han J, Ye M, Xu M, et al. Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 2007; 848(2): 355-362. doi: 10.1016/j.jchromb.2006.10.061 [151] Zhang Y, Wang X, Wang X, et al. Protective effect of flavonoids from Scutellaria baicalensis Georgi on cerebral ischemia injury. J Ethnopharmacol, 2006; 108(3): 355-360. doi: 10.1016/j.jep.2006.05.022 [152] Ku S K, Lee I C, Bae J S. Antithrombotic activities of oroxylin A in vitro and in vivo. Arch Pharm Res, 2014; 37: 679-686. doi: 10.1007/s12272-013-0233-0 [153] Lee W, Ku S K, Bae J S. Antiplatelet, anticoagulant, and profibrinolytic activities of baicalin. Arch Pharm Res, 2015; 38(5): 893-903. doi: 10.1007/s12272-014-0410-9 [154] Ku S K, Bae J S. Antithrombotic activities of wogonin and wogonoside via inhibiting platelet aggregation. Fitoterapia, 2014; 98: 27-35. doi: 10.1016/j.fitote.2014.07.006 [155] Liu X F, Liu M L, Iyanagi T, et al. Inhibition of rat liver NAD(P)H: quinone acceptor oxidoreductase (DT-diaphorase) by flavonoids isolated from the Chinese herb scutellariae radix (Huang Qin). Mol Pharmacol, 1990; 37(6): 911-915. http://www.ncbi.nlm.nih.gov/pubmed/1694261/ [156] Wu Y H, Chuang L P, Yu C L, et al. Anticoagulant effect of wogonin against tissue factor expression. Eur J Pharmacol, 2019; 859: 172517. doi: 10.1016/j.ejphar.2019.172517 [157] Zhang X, Jin M, Tadesse N, et al. Dioscorea zingiberensis C. H. Wright: An overview on its traditional use, phytochemistry, pharmacology, clinical applications, quality control, and toxicity. J Ethnopharmacol, 2018; 220: 283-293. doi: 10.1016/j.jep.2018.03.017 [158] Li H, Huang W, Wen Y, et al. Anti-thrombotic activity and chemical characterization of steroidal saponins from dioscorea zingiberensis C.H. Wright. Fitoterapia, 2010; 81(1): 1147-1156. http://www.onacademic.com/detail/journal_1000035046189810_1031.html [159] Gong G, Qin Y, Huang W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis C.H. Wright in vitro and in vivo. Phytomedicine, 2011; 18(6): 458-463. doi: 10.1016/j.phymed.2010.08.015 [160] Zheng H, Wei Z, Xin G, et al. Preventive effect of a novel diosgenin derivative on arterial and venous thrombosis in vivo. Bioorg Med Chem Lett, 2016; 26(14): 3364-3369. doi: 10.1016/j.bmcl.2016.05.032 [161] Chen Y, Chen P D, Bao B H, et al. Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. J Ethnopharmacol, 2018; 219: 152-160. doi: 10.1016/j.jep.2017.11.005 [162] Shan M, Yu S, Yan H, et al. A review of the botany, phytochemistry, pharmacology and toxicology of rubiae radix et rhizoma. Molecules, 2016; 21(12): 1747. doi: 10.3390/molecules21121747 [163] Nishimura N, Takai M, Yamamoto E, et al. Purpurin as a specific inhibitor of spermidine-induced autoactivation of the protease plasma hyaluronan-binding protein. Biol Pharm Bull, 2010; 33(8): 1430-1433. doi: 10.1248/bpb.33.1430 [164] Auyeung K K, Han Q B, Ko J K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med, 2016; 44(1): 1-22. doi: 10.1142/S0192415X16500014 [165] Zhao J, Yang P, Li F, et al. Therapeutic effects of astragaloside IV on myocardial injuries: multi-target identification and network analysis. PLoS One, 2012; 7(9): e44938. doi: 10.1371/journal.pone.0044938 [166] Zhang W J, Wojta J, Binder B R. Regulation of the fibrinolytic potential of cultured human umbilical vein endothelial cells: astragaloside IV downregulates plasminogen activator inhibitor-1 and upregulates tissue-type plasminogen activator expression. J Vasc Res, 1997; 34(4): 273-280. doi: 10.1159/000159234 [167] Koo Y K, Kim J M, Koo J Y, et al. Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa. Pharmazie, 2010; 65(8): 624-628. http://www.ncbi.nlm.nih.gov/pubmed/20824965 [168] Xie P, Cui L, Shan Y, et al. Antithrombotic effect and mechanism of radix paeoniae rubra. Biomed Res Int, 2017; 17: 9475074. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5337344/pdf/BMRI2017-9475074.pdf [169] Ye J, Duan H, Yang X, et al. Anti-thrombosis effect of paeoniflorin: evaluated in a photochemical reaction thrombosis model in vivo. Planta Med, 2001; 67(8): 766-767. doi: 10.1055/s-2001-18364 [170] Ngo T, Kim K, Bian Y, et al. Antithrombotic effects of paeoniflorin from paeonia suffruticosa by selective inhibition on shear stress-induced platelet aggregation. Int J Mol Sci, 2019; 20(20): 5040. doi: 10.3390/ijms20205040 [171] Ye S, Mao B, Yang L, et al. Thrombosis recanalization by paeoniflorin through the upregulation of urokinasetype plasminogen activator via the MAPK signaling pathway. Mol Med Rep, 2016; 13(6): 4593-4598. doi: 10.3892/mmr.2016.5146 [172] Kocaadam B, Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr, 2017; 57(13): 2889-2895. doi: 10.1080/10408398.2015.1077195 [173] Srivastava K C, Bordia A, Verma S K. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot Essent Fatty Acids, 1995; 52(4): 223-227. doi: 10.1016/0952-3278(95)90040-3 [174] Lee H S. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour Technol, 2006; 97(12): 1372-1376. doi: 10.1016/j.biortech.2005.07.006 [175] Manikandan P, Sumitra M, Aishwarya S, et al. Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int J Biochem Cell Biol, 2004; 36(10): 1967-1980. doi: 10.1016/j.biocel.2004.01.030 [176] Heemskerk J W, Sage S O. Calcium signalling in platelets and other cells. Platelets, 1994; 5(6): 295-316. doi: 10.3109/09537109409006439 [177] Mayanglambam A, Dangelmaier C A, Thomas D, et al. Curcumin inhibits GPVI-mediated platelet activation by interfering with the kinase activity of Syk and the subsequent activation of PLCgamma2. Platelets, 2010; 21(3): 211-220. doi: 10.3109/09537100903528269 [178] Xia Q, Wang X, Xu D J, et al. Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil. Thromb Res, 2012; 130(3): 409-414. doi: 10.1016/j.thromres.2012.04.005 [179] Kim D C, Ku S K, Bae J S. Anticoagulant activities of curcumin and its derivative. BMB Rep, 2012; 45(4): 221-226. doi: 10.5483/BMBRep.2012.45.4.221 [180] Koizume S, Yokota N, Miyagi E, et al. Hepatocyte nuclear factor-4-independent synthesis of coagulation factor VⅡ in breast cancer cells and its inhibition by targeting selective histone acetyltransferases. Mol Cancer Res, 2009; 7(12): 1928-1936. doi: 10.1158/1541-7786.MCR-09-0372 [181] Madhyastha R, Madhyastha H, Nakajima Y, et al. Curcumin facilitates fibrinolysis and cellular migration during wound healing by modulating urokinase plasminogen activator expression. Pathophysiol Haemost Thromb, 2010; 37(2-4): 59-66. http://www.researchgate.net/profile/Harishkumar_Madhyastha/publication/47755863_Curcumin_facilitates_fibrinolysis_and_cellular_migration_during_wound_healing_by_modulating_urokinase_plasminogen_activator_expression/links/540fa7710cf2d8daaad0a8e0.pdf [182] Grau T, Vilcinskas A, Joop G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z Naturforsch C J Biosci, 2017; 72(9): 337-349. http://www.onacademic.com/detail/journal_1000039908383210_a734.html [183] Lee W, Kim M A, Park I, et al. Novel direct factor Xa inhibitory compounds from Tenebrio molitor with anti-platelet aggregation activity. Food Chem Toxicol, 2017; 109(Pt 1): 19-27. http://www.onacademic.com/detail/journal_1000040063287110_7e0f.html -


 投稿系统
投稿系统


 下载:
下载: