Association of dietary phytosterols with prevalence of metabolic dysfunction-associated fatty liver disease in adult population of Northeastern China: An internet-based cross-sectional study
doi: 10.1515/fzm-2025-0005
-
Abstract:
Objective The benefits of phytosterols have attracted growing interest, but their association with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) has rarely been reported in population-based studies. This research aimed to investigate the correlation between dietary phytosterols and MAFLD. Methods Phytosterols intake was evaluated using an internet-based dietary questionnaire targeted at the Chinese population. Conditional logistic regression models were employed to investigate dose-response relationships between phytosterol intake and MAFLD, as well as the potential preventive role of phytosterols. Restricted Cubic Spline (RCS) analyses were conducted to examine associations between phytosterols intake and MAFLD. Additionally, a quantile-based g-computation (qgcomp) method was applied to explore the combined effect of campesterol, stigmasterol, β-sitostelane, campestane, and β-sitosterol on MAFLD. Results Significant inverse relationships were found between total phytosterols and MAFLD (OR, 0.19; 95% CI, 0.11-0.32; P < 0.001), campesterol (OR, 0.22; 95% CI, 0.13-0.37; P < 0.001), stigmasterol (OR, 0.17; 95% CI, 0.10-0.30; P < 0.001), β-sitostelane (OR, 0.26; 95% CI, 0.16-0.45; P < 0.001), campestane (OR, 0.23; 95% CI, 0.14-0.39; P < 0.001), and β-sitosterol (OR, 0.17; 95% CI, 0.10-0.29; P < 0.001). The qgcomp analysis showed a significant negative association between the five phytosterols and MAFLD (OR, 0.58; 95% CI, 0.50-0.67; P < 0.001). Additionally, the qgcomp analysis revealed that the combination of these five phytosterols was inversely associated with MAFLD, with stigmasterol contributing the most (weight = 0.70). Conclusion Higher intake of phytosterols was associated with a reduced prevalence of MAFLD, with stigmasterol showing the most significant inverse relationship. Further research is needed to clarify the relationship between phytosterols and MAFLD. -
Figure 3. Restricted multivariable cubic spline plots illustrating the association between phytosterols intake and the risk of MAFLD
(A) Total phytosterols; (B) Campesterol; (C) Sigmasterol; (D) β-Sitostelane; (E) Campestane; (F) β-Sitosterol. All models were adjusted for energy intake, age, gender, BMI, alcohol consumption, smoking, work intensity, education level, and income.
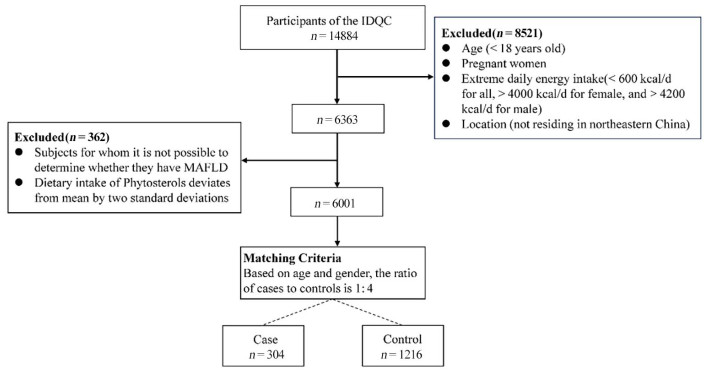
Table 1. Basic demographic information and nutrients intake of the subjects
Variable Control (n = 1216) MAFLD (n = 304) P value Demographic factors Age, year 53.01 ± 12.59 52.98 ± 12.57 0.97 Male, n (%) 456 (37.50) 114 (37.50) Female, n (%) 740 (66.07) 185 (66.07) BMI, kg/m2 24.11 ± 2.96 27.22 ± 3.59 < 0.01 Smoking, (%current) 164 (13.49) 30(9.87) 0.11 Drinking, (%current) 191 (15.71) 25(8.22) < 0.01 Income per month, Yuan, n (%) < 0.01 < 1000 221 (18.17) 52(17.11) 1000-2000 296 (24.34) 58(19.08) 2000-3000 359 (29.52) 77(25.33) 3000-4000 192 (15.79) 42(13.82) 4000-5000 88 (7.24) 27(8.88) 5000-6000 33 (2.71) 26(8.55) > 6000 27 (2.22) 22(7.24) Education levels, n (%) < 0.01 Junior school and below 533 (43.83) 94(30.92) Senior high school or equivalent 257 (21.13) 78(25.66) College or equivalent 399 (32.81) 117 (38.49) Postgraduate or above 27 (2.22) 15(4.93) Work intensity, n (%) Light 780 (64.14) 249 (81.91) < 0.001 Medium 168 (13.82) 31(10.20) Heavy 268 (22.04) 24(7.89) Dietary factors Energy, kcal/day 2150.37 ± 773.51 2072.02 ± 707.80 0.11 Protein, g/day 80.46 ± 14.75 82.75 ± 16.68 < 0.05 Fat, g/day 66.00 ± 20.75 62.38 ± 19.75 < 0.01 Carbohydrates, g/day 311.41 ± 52.13 318.08 ± 46.88 < 0.05 β-Sitosterol, mg/day 629.26 ± 548.63 453.11 ± 553.48 < 0.01 Campesterol, mg/day 63.20 ± 44.14 48.76 ± 43.94 < 0.01 Stigmasterol, mg/day 60.99 ± 37.88 44.61 ± 39.16 < 0.01 β-Sitostelane, mg/day 65.69 ± 75.18 48.66 ± 70.32 < 0.01 Campestane, mg/day 164.43 ± 193.50 120.61 ± 179.37 < 0.01 Total phytosterols, mg/day 1009.44 ± 813.08 725.43 ± 846.66 < 0.01 The P values were determined using the Chi-squared test for categorical variables, Student's t-test for continuous variables. Data were expressed as mean ± SD, and as frequencies and percentages where appropriate; MAFLD: non-alcoholic fatty liver disease. -
[1] Vernon G, Baranova A, Younossi Z. M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther, 2011; 34(3): 274-285. doi: 10.1111/j.1365-2036.2011.04724.x [2] Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology, 2019; 69(6): 2672-2682. doi: 10.1002/hep.30251 [3] Pais R, Barritt A S T, Calmus Y, et al. MAFLD and liver transplantation: Current burden and expected challenges. J Hepatol, 2016; 65(6): 1245-1257. doi: 10.1016/j.jhep.2016.07.033 [4] Huebbe P, Bilke S, Rueter J, et al. Human APOE4 protects high-fat and high-sucrose diet fed targeted replacement mice against fatty liver disease compared to APOE3. Aging Dis, 2024; 15(1): 259-281. doi: 10.14336/AD.2023.0530 [5] Paik J. M, Golabi P, Younossi Y, et al. The growing burden of disability related to nonalcoholic fatty liver disease: data from the global burden of disease 2007-2017. Hepatol Commun, 2020; 4(12): 1769-1780. doi: 10.1002/hep4.1599 [6] Golabi P, Paik J M, Alqahtani S, et al. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: data from global burden of disease 2009-2019. J Hepatol, 2021; 75(4): 795-809. doi: 10.1016/j.jhep.2021.05.022 [7] Adams L A, Anstee Q M, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut, 2017; 66(6): 1138-1153. doi: 10.1136/gutjnl-2017-313884 [8] Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology, 2005; 42(1): 44-52. doi: 10.1002/hep.20734 [9] Liu S, Liu Y, Wan B, et al. Association between Vitamin D status and non-alcoholic fatty liver disease: a population-based study. J Nutr Sci Vitaminol (Tokyo), 2019; 65(4): 303-308. doi: 10.3177/jnsv.65.303 [10] Machado V A, Santisteban A R N, Martins C M, et al. Effects of phytosterol supplementation on lipoprotein subfractions and LDL particle quality. Sci Rep, 2024; 14(1): 11108. doi: 10.1038/s41598-024-61897-4 [11] Nattagh-Eshtivani E, Barghchi H, Pahlavani N, et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother Res, 2022; 36(1): 299-322. doi: 10.1002/ptr.7312 [12] Cusack L K, Fernandez M L, Volek J S. The food matrix and sterol characteristics affect the plasma cholesterol lowering of phytosterol/ phytostanol. Adv Nutr, 2013; 4(6): 633-643. doi: 10.3945/an.113.004507 [13] Ding X, Xu Y, Nie P, et al. Changes in the serum metabolomic profiles of subjects with MAFLD in response to n-3 PUFAs and phytosterol ester: a double-blind randomized controlled trial. Food Funct, 2022; 13(9): 5189-5201. doi: 10.1039/D1FO03921K [14] Song L, Qu D, Zhang Q, et al. Phytosterol esters attenuate hepatic steatosis in rats with non-alcoholic fatty liver disease rats fed a high-fat diet. Sci Rep, 2017; 7: 41604. doi: 10.1038/srep41604 [15] Friedman S L, Neuschwander-Tetri B A, Rinella M, et al. Mechanisms of MAFLD development and therapeutic strategies. Nat Med, 2018; 24(7): 908-922. doi: 10.1038/s41591-018-0104-9 [16] Miettinen T A, Tilvis R S, Kesäniemi Y A. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol, 1990; 131(1): 20-31. doi: 10.1093/oxfordjournals.aje.a115479 [17] Plat J, Hendrikx T, Bieghs V, et al. Protective role of plant sterol and stanol esters in liver inflammation: insights from mice and humans. PLoS One, 2014; 9(10): e110758. doi: 10.1371/journal.pone.0110758 [18] Sánchez-Crisóstomo I, Fernández-Martínez E, Cariño-Cortés R, et al. Phytosterols and triterpenoids for prevention and treatment of metabolic-related liver diseases and hepatocellular carcinoma. Curr Pharm Biotechnol, 2019; 20(3): 197-214. doi: 10.2174/1389201020666190219122357 [19] Xia M, Sun X, Zheng L, et al. Regional difference in the susceptibility of non-alcoholic fatty liver disease in China. BMJ Open Diab Res Ca, 2020; 8(1): e001311. doi: 10.1136/bmjdrc-2020-001311 [20] Jain R, Wade G, Ong I, et al. Determination of tissue contributions to the circulating lipid pool in cold exposure via systematic assessment of lipid profiles. J Lipid Res, 2022; 63(7): 100197. doi: 10.1016/j.jlr.2022.100197 [21] Song Z, Yang H, Huang X, et al. The spatiotemporal pattern and influencing factors of land surface temperature change in China from 2003 to 2019. Int J Appl Earth Obs Geoinf, 2021, 104: 102537. [22] Ji X N, Huang M, Yao S H, et al. Refined grains intake in high fat, high protein, low carbohydrate and low energy levels subgroups and higher likelihood of abdominal obesity in Chinese population. Int J Food Sci Nutr, 2020; 71(8): 979-990. doi: 10.1080/09637486.2020.1746956 [23] Feng R N, Du S S, Chen Y, et al. An internet-based food frequency questionnaire for a large Chinese population. Asia Pac J Clin Nutr, 2016; 25(4): 841-848. [24] Li Y C, Li C L, Qi J Y, et al. Relationships of dietary histidine and obesity in northern chinese adults, an internet-based cross-sectional study. Nutrients, 2016; 8(7). doi: 10.3390/nu8070420 [25] Uhlig C E, Seitz B, Eter N, et al. Efficiencies of Internet-based digital and paper-based scientific surveys and the estimated costs and time for different-sized cohorts. PLoS One, 2014; 9(10): e108441. doi: 10.1371/journal.pone.0108441 [26] Eslam M, Newsome P. N, Sarin S. K, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol, 2020; 73(1): 202-209. doi: 10.1016/j.jhep.2020.07.045 [27] Keil A. P, Buckley J P, O'brien K M, et al. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect, 2020; 128(4): 47004. doi: 10.1289/EHP5838 [28] Simón J, Casado-Andrés M, Goikoetxea-Usandizaga N, et al. Nutraceutical properties of polyphenols against liver diseases. Nutrients, 2020; 12(11). doi: 10.3390/nu12113517 [29] Li Y C, Li C. L, Li R, et al. Associations of dietary phytosterols with blood lipid profiles and prevalence of obesity in Chinese adults, a cross-sectional study. Lipids Health Dis, 2018; 17(1): 54. doi: 10.1186/s12944-018-0703-y [30] Kruse M, Kemper M, Gancheva S, et al. Dietary rapeseed oil supplementation reduces hepatic steatosis in obese men-a randomized controlled trial. Mol Nutr Food Res, 2020; 64(21): e2000419. doi: 10.1002/mnfr.202000419 [31] Kuwabara M, Sasaki J, Ouchi Y, et al. Higher cholesterol absorption marker at baseline predicts fewer cardiovascular events in elderly patients receiving hypercholesterolemia treatment: The KEEP Study. J Am Heart Assoc, 2024; 13(3): e031865. doi: 10.1161/JAHA.123.031865 [32] Jordão Candido C, Silva Figueiredo P, Del Ciampo Silva R, et al. Protective effect of α-linolenic acid on non-alcoholic hepatic steatosis and interleukin-6 and -10 in wistar rats. Nutrients, 2019; 12(1). doi: 10.3390/nu12010009 [33] Wang X, Wang Y, Xu W, et al. Dietary isoflavones intake is inversely associated with non-alcoholic fatty liver disease, hyperlipidaemia and hypertension. Int J Food Sci Nutr, 2022; 73(1): 60-70. doi: 10.1080/09637486.2021.1910630 [34] Song L, Zhao X G, Ouyang P L, et al. Combined effect of n-3 fatty acids and phytosterol esters on alleviating hepatic steatosis in nonalcoholic fatty liver disease subjects: a double-blind placebo-controlled clinical trial. Br J Nutr, 2020; 123(10): 1148-1158. doi: 10.1017/S0007114520000495 [35] Li X, Xin Y, Mo Y, et al. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules, 2022; 27(2): 523. doi: 10.3390/molecules27020523 [36] Lifsey H C, Kaur R, Thompson B H, et al. Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine. J Nutr Biochem, 2020; 76: 108263. doi: 10.1016/j.jnutbio.2019.108263 [37] Xin Y, Li X, Zhu X, et al. Stigmasterol protects against steatohepatitis induced by high-fat and high-cholesterol diet in mice by enhancing the alternative bile acid synthesis pathway. J Nutr, 2023; 153(7): 1903-1914. doi: 10.1016/j.tjnut.2023.05.026 [38] Jayaraman S, Devarajan N, Rajagopal P, et al. β-sitosterol circumvents obesity induced inflammation and insulin resistance by down-regulating IKKβ/NF-κB and JNK signaling pathway in adipocytes of type 2 diabetic rats. Molecules, 2021; 26(7): 2101. doi: 10.3390/molecules26072101 [39] Vezza T, Canet F, De Marañón A M, et al. Phytosterols: nutritional health players in the management of obesity and its related disorders. Antioxidants (Basel), 2020; 9(12): 1266. doi: 10.3390/antiox9121266 [40] Desai A J, Dong M, Miller L J. Beneficial effects of β-sitosterol on type 1 cholecystokinin receptor dysfunction induced by elevated membrane cholesterol. Clin Nutr, 2016; 35(6): 1374-1379. doi: 10.1016/j.clnu.2016.03.003 [41] Zhao J, Wang C, Shi X, et al. Modeling climatically suitable areas for soybean and their shifts across China. Agric Syst, 2021, 192: 103205. [42] Wang G X, Ma Q H, Zhao T T, et al. Resources and production of hazelnut in China. Acta Horticulturae, 2018; (1226): 59-64. [43] Han H, Xue T, Li J, et al. Plant sterol ester of α-linolenic acid improved non-alcoholic fatty liver disease by attenuating endoplasmic reticulum stress-triggered apoptosis via activation of the AMPK. J Nutr Biochem, 2022; 107: 109072. doi: 10.1016/j.jnutbio.2022.109072 -


 投稿系统
投稿系统


 下载:
下载: